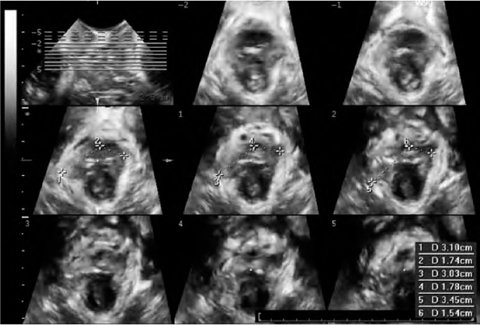
Fig. 6.1
Two-dimensional transperineal ultrasonography. Midsagittal view of normal female pelvic floor, including the symphysis pubis, the urethra and bladder, the vagina and uterus, the rectum, and the anal canal. Posterior to the anorectal junction, the puborectalis (PR) muscle is visualized as a hyperechogenic structure
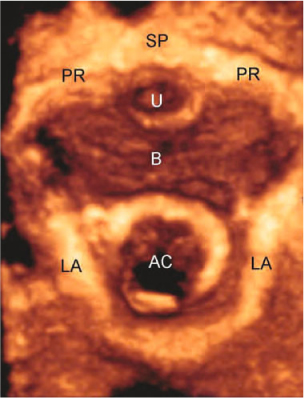
Fig. 6.2
Three-dimensional transperineal ultrasonography. Axial view of normal female pelvic floor, including the symphysis pubis (SP) and pubic rami (PR), the urethra (U) and bladder (B), the levator ani (LA), and the anal canal (AC)
6.2.2 Endovaginal Ultrasonography
Endovaginal ultrasonography (EVUS) is performed with the patient placed in the same position as that adopted for TPUS. It may be performed with a high multifrequency (9–16 MHz), 360° rotational mechanical probe (type 2050, B-K Medical, Herlev, Denmark) or with a radial electronic probe (type AR 54 AW, 5–10 MHz, Hitachi Medical Systems) [8]. The difference between these two transducers is that the 3D acquisition is free-hand with the electronic transducer, whereas the mechanical transducer has an internal automated motorized system that allows an acquisition of 300 aligned transaxial 2D images over a distance of 60 mm in 60 s, without any movement of the probe within the tissue. The set of 2D images is reconstructed instantaneously into a high-resolution 3D image for real-time manipulation and volume rendering. An advantage of 3D compared with the 2D mode is the opportunity to obtain sagittal, axial, coronal, and any desired oblique sectional image. The 3D image may be rotated, tilted, and sliced to allow the operator to vary infinitely the different section parameters, and to visualize and measure distance, area, angle, and volume in any plane. The 3D volume can also be archived for offline analysis on the ultrasonographic system or on a PC with the help of dedicated software [8].
6.2.3 Endoanal Ultrasonography
Endoanal ultrasonography (EAUS) is performed with the same probes adopted for EVUS [9]. During examination, the patient may be placed in a dorsal lithotomy, left lateral, or prone position. However, irrespective of patient position, the transducer should be rotated so that the anterior aspect of the anal canal is superior (12 o’clock position) on the screen, the right lateral aspect is to the left (9 o’clock), the left lateral aspect is to the right (3 o’clock), and the posterior aspect is inferior (6 o’clock). The recording of data should extend from the upper aspect of the PR muscle to the anal verge. The mechanical rotational transducer allows automatic 3D acquisition.
6.3 Ultrasonographic Anatomy
Evaluation of the complex anatomy and function of the pelvic floor may require more than one ultrasonographic modality. TPUS, EVUS, and EAUS may provide complementary information, and often multicompartmental scanning is needed to obtain a complete overview [3].
6.3.1 Pelvic Floor Structures
3D-EVUS performed with 360° field-of-view transducers provides a topographical overview of pelvic floor anatomy [8]. Four levels of assessment in the axial plane can be defined (Fig. 6.3). At the highest level (level I), the bladder base can be seen anteriorly and the inferior third of the rectum posteriorly. Level II corresponds to the bladder neck, the intramural region of the urethra, and the anorectal junction. Level III corresponds to the midurethra and the upper third of the anal canal. At this level, the levator ani can be visualized as a multilayer hyperechoic sling coursing laterally to the vagina and posteriorly to the anal canal, and attaching to the inferior pubic rami anteriorly. Biometric indices of the levator hiatus can be measured [11]: (a) the anteroposterior diameter, from the inferior border of the symphysis pubis to the 6 o’clock inner margin of the levator ani; (b) the laterolateral diameter, measured at the widest part, perpendicular to the anteroposterior diameter; (c) and the area, calculated as the area within the levator ani inner perimeter enclosed by the inferior pubic rami and the inferior edge of the symphysis pubis. At the lowest level (level IV), the superficial perineal muscles (bulbospongiosus, ischiocavernosus, and superficial transverse perineal muscles), the perineal body, the distal urethra, and the middle and inferior thirds of the anal canal can be visualized. At this level, the anteroposterior diameter of the urogenital hiatus, corresponding to the symphysis pubis-perineal body distance, can be determined.
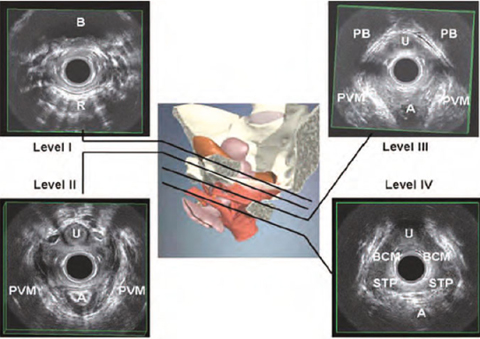

Fig. 6.3
Three-dimensional endovaginal ultrasonography. Four standard levels of assessment of the normal female pelvic floor: levels I-IV. A, anal canal; B, bladder; BCM, bulbocavernosus muscle; PB, pubic bone; PVM, pubovisceral muscle; R, rectum; STP, superficial transverse perinei muscle; U, urethra. (Reproduced from [10], with permission)
Pelvic organ descent is usually assessed with 2D-TPUS [6]. A midsagittal view, obtained with an acquisition angle of 70° or more, will include the symphysis pubis, the urethra and bladder, the vagina and uterus, the rectum, and the anal canal. Posterior to the anorectal junction, the PR muscle is visualized as a hyperechogenic structure (Fig. 6.1). 3D-TPUS provides the following additional information in the reconstructed axial plane [7] (Fig. 6.2): levator hiatus dimensions, determined in the plane of minimum anteroposterior dimensions; PR muscle dimensions, determined in the plane of maximum muscle thickness; qualitative assessment of the PR muscle and its insertion on the inferior pubic ramus. Biometric indices of the levator hiatus at rest determined in nulliparous women by Santoro et al. [11] using 3D-EVUS (anteroposterior diameter 4.84 cm, laterolateral diameter 3.28 cm, hiatal area 12 cm2) were comparable to the results reported by Dietz et al. [7] using 3D-TPUS (anteroposterior diameter 4.52 cm, laterolateral diameter 3.75 cm, hiatal area 11.25 cm2). The main advantage of external (transperineal) over internal (endovaginal) sonographic imaging of the levator ani is that maneuvers such as Valsalva and pelvic floor muscle contraction allow its functional assessment. Ballooning of the hiatus, i.e., excessive distensibility of the levator ani, is defined as an increase in hiatal area to > 25 cm2 on Valsalva maneuver, and its visualization generally requires full development of pelvic organ prolapse (POP) [12]. Quantitative assessment of muscle trauma is greatly facilitated by tomographic ultrasound [13].
6.3.2 Anterior Compartment
Assessment of the anterior compartment is performed with 2D-TPUS [4] (Fig. 6.1). Measurements in the midsagittal plane, performed with the patient at rest or during functional maneuvers, include [14]: bladder wall thickness or detrusor wall thickness (normal value, up to 5 mm); postvoid residual bladder volume; bladder-symphysis distance, measured between the bladder neck and the lower margin of the symphysis pubis (this measurement enables assessment of the position and mobility of the bladder neck, using the difference between values obtained at rest and on Valsalva; there is no definition of ‘normal’ for bladder neck descent, although a cut-off of 25 mm has been proposed to define hypermobility); urethral length, measured from the bladder neck to the external urethral orifice; retrovesical angle, the angle between the posterior wall of the bladder and the longitudinal axis of the urethra (normal value, 90–120°); proximal urethral rotation (change in angle between proximal urethra and central symphyseal axis) on Valsalva; and descent of the most inferior aspect of a cystocele relative to the symphysis pubis.
6.3.3 Central Compartment
Assessment of the central compartment is performed with 2D-TPUS [4] (Fig. 6.1). In the midsagittal section, an unusually low cervix is isoechoic, its distal margin evident as a specular line, and it often causes acoustic shadowing. In the same section, it is possible to visualize the corpus uteri and determine whether it is enlarged, retroverted, or anteverted. Dynamic 2D-TPUS allows evaluation of uterine descent that may result in compression of the rectal ampulla, explaining symptoms of obstructed defecation. Imaging of vault descent is more difficult, because the vaginal wall is often obscured by a descending rectocele or enterocele.
6.3.4 Posterior Compartment
The anal canal is generally imaged by 3D-EAUS [9]. This method is firmly established as one of the cornerstones of a colorectal diagnostic work-up [15]. With EAUS, the anal canal is divided into three levels of assessment in the axial plane (Fig. 6.4). (1) The upper level corresponds to the hyperechoic sling of the PR muscle and the concentric hypoechoic ring of the internal anal sphincter (IAS). In males, the deep part of the external anal sphincter (EAS) is also identified at this level. (2) In the middle level, the complete ring of the superficial EAS (concentric band of mixed echogenicity), the conjoined longitudinal layer, the complete ring of the IAS and the transverse perinei muscles are visualized. (3) The lower level corresponds to the subcutaneous part of the EAS. 3D-EAUS is useful in assessing the anatomical characteristics of the anal canal [9]. The muscles of the lower and upper parts of the anal canal are different. At its upper end, the PR muscle anchors the sphincter complex to the pubic rami. Anteriorly, the circular fibers of the deep part of the EAS are not recognizable in females, whereas in males the EAS is symmetrical at all levels of the anal canal. The IAS is not completely symmetrical, either in thickness or at its distal end. It can be traced superiorly into the circular muscle of the rectum, extending from the anorectal junction to approximately 1 cm below the dentate line. In the intersphincteric space, the smooth longitudinal muscle conjoins with striated muscle fibers from the levator ani, particularly the puboanalis, and a large fibroelastic element derived from the endopelvic fascia to form the conjoined longitudinal layer.
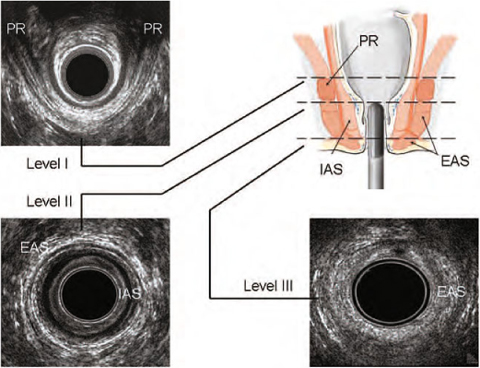

Fig. 6.4
Three-dimensional endoanal ultrasonography. Three standard levels of assessment of the normal anal canal: levels I-III. EAS, external anal sphincter; IAS, internal anal sphincter; PR, puborectalis muscle. (Reproduced from [10], with permission)
6.4 Clinical Applications
6.4.1 Urinary Incontinence
Urinary incontinence has been defined by the International Urogynecology Association and the International Continence Society as ‘involuntary loss of urine’ [16]. This condition is exceptionally common, with more than 40% of women over the age of 40 years estimated to experience it. The most common types are: stress urinary incontinence (SUI), defined as involuntary loss of urine during increased abdominal pressure, thought to be due to a poorly functioning urethral sphincter muscle (intrinsic sphincter deficiency) or to hypermobility of the bladder neck or urethra; and urge urinary incontinence (UUI), defined as involuntary urinary leakage accompanied or immediately preceded by urgency, due to detrusor overactivity [16]. Ultrasonography can provide essential information in the management of SUI [5]. Tunn et al. [17] recommended measurement of the retrovesical angle with TPUS in patients with SUI. For quantitative evaluation of urethral mobility, Valsalva maneuver is preferable to the cough test. In patients with SUI or UUI, funneling of the internal urethral meatus may be observed on Valsalva and sometimes even at rest. Marked funneling has been shown to be associated with poor urethral closure pressures. TPUS allows comprehensive evaluation of many abnormalities of the female urethra, such as urethral diverticula, abscesses, tumors, and other urethral and paraurethral lesions [5].
Ultrasonography also allows evaluation of tapes used in anti-incontinence surgery, whose improper positioning or dislodgement may be associated with failed surgery (Fig. 6.5). Dietz et al. [18] performed 3D-TPUS to assess the effectiveness of suburethral slings (tension-free vaginal tape, intravaginal slingplasty, and suprapubic arch sling system). All three tapes were visualized by ultrasound and showed comparable short-term clinical and anatomical outcomes. Ultrasound is particularly useful in the assessment of postoperative voiding dysfunction. The minimum gap between implant and symphysis pubis on maximal Valsalva maneuver seems to be the single most useful parameter in the postoperative evaluation of suburethral tapes, as it is associated negatively with voiding dysfunction and positively with both SUI and UUI. Occasionally, sonographic findings will suggest tape perforation (partial or complete), with the implant found within the rhabdosphincter muscle, or even crossing the urethral lumen. At times it is necessary to divide an obstructive tape, and ultrasound can help in locating the tape, as well as in confirming tape division postoperatively.
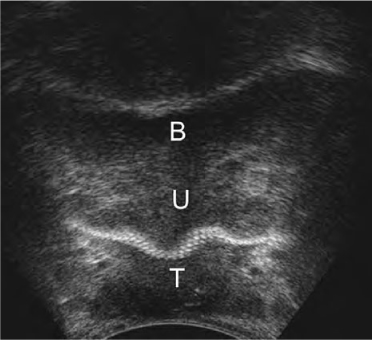

Fig. 6.5
Two-dimensional transperineal ultrasonography. Midurethra (U) tape (T). B, bladder
6.4.2 Fecal Incontinence
Fecal incontinence (FI) is defined as the involuntary loss of feces (liquid or solid stool), and anal incontinence is defined as the involuntary loss of flatus or feces [16]. Intact musculature, including the PR muscle, IAS, and EAS, is a prerequisite for fecal control, as is a functioning nerve supply to these muscles. Other factors contributing to continence include stool consistency, rectal sensitivity and capacity, and anorectal angle. Any impairment of one or more of these factors may result in FI. Anal sphincter defects and pudendal nerve damage occurring during vaginal delivery are by far the most common causes of FI, consequently making this problem more prevalent in women than men. In patients with FI, it is fundamental to establish the underlying pathophysiology in order to choose the appropriate therapy (e.g., dietary adjustments, medication, biofeedback, sphincter repair, artificial bowel sphincter, graciloplasty, sacral nerve stimulation, and injection of bulking agents) [19]. EAUS has become the gold standard for morphological assessment of the anal canal [15]. It can differentiate between incontinent patients with intact anal sphincters and those with sphincter lesions (defects, scarring, thinning, thickening, and atrophy) [9]. Tears are identified by interruption of the circumferential fibrillar echo texture (Fig.6.6). Scarring is characterized by loss of normal architecture, with an area of amorphous texture that usually has low reflectivity. Two scoring systems have been proposed to define the severity of anal sphincter damage. Starck et al. [20] introduced a specific score, with 0 indicating no defect and 16 corresponding to a defect >180° involving the whole length and depth of both sphincters. Noderval et al. [21] described a simplified system for analyzing defects: the maximal score of 7 denotes defects in both the EAS and the IAS exceeding 90° in the axial plane and involving more than half of the length of each sphincter. The presence of a sphincter defect, however, does not necessarily mean that it is the cause of the FI, as many people have sphincter lesions without having symptoms of incontinence. On the other hand, patients with FI and an apparently intact sphincter may have muscle degeneration, atrophy, or pudendal neuropathy. Ultrasonography also allows evaluation of anti-incontinence surgery (sphincter repair, graciloplasty, bulking agent injection) [3].
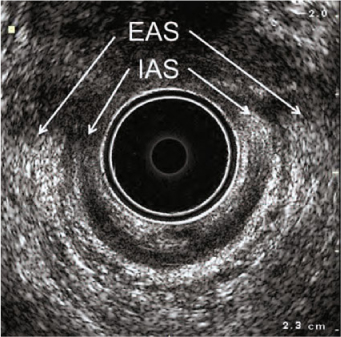

Fig. 6.6
Three-dimensional endoanal ultrasonography. Combined anterior damage of the external (EAS) and internal anal sphincters (IAS) from the 10 o’clock to the 2 o’clock position
6.4.3 Levator Ani Injuries
Levator avulsion is the disconnection of the muscle from its insertion on the inferior pubic ramus and the pelvic sidewall, whereas tears may occur in any part of the muscle. Avulsion is a common consequence of overstretching of the levator ani during the second stage of labor and occurs in 10–36% of women at the time of their first delivery [21]. 3D-EVUS and 3D-TPUS may be utilized to document major levator trauma [3, 12, 13] (Fig. 6.7). Defects are usually visualized most clearly on maximal pelvic floor muscle contraction.