In the early part of the 20th century, many research groups were trying to isolate insulin from the other digestive proteins of the pancreas. In 1921, Fredrick Banting and his assistant Charles Best succeeded in purifying insulin and treating diabetes. For the first time, patients with type 1 diabetes could live well into adulthood. In 1966, the world’s first simultaneous kidney and pancreas transplant was performed on a 28-year-old woman with diabetes and renal failure at the University of Minnesota by Kelly and Lillehei. In the subsequent 45 years, simultaneous pancreas and kidney transplantation has been adopted as the definitive treatment for type 1 diabetes complicated by end-stage renal disease. Pancreas transplantation is also used to treat type 1 diabetes without renal failure in patients who have life-threatening complications, such as severe hypoglycemic unawareness. Increasingly, pancreas transplantation is being recognized as a beneficial treatment for nonobese patients with type 2 diabetes and its attendant complications. Approximately 6% of pancreas transplants are performed for treatment of type 2 diabetes.
Pancreas transplant is typically performed in combination with kidney transplantation (simultaneous pancreas and kidney, or SPK) but can also be performed after kidney transplant (pancreas after kidney, or PAK) or in isolation (pancreas alone, or PA). The goal of pancreas transplantation is exogenous insulin-free euglycemia. Intensive control of blood sugars with insulin administration can delay the onset and slow the progression of diabetic complications but cannot reverse them. On the other hand, pancreas transplantation offers the prospect of excellent glycemic control free of exogenous insulin administration, improvement in quality of life, and reduction in diabetes-associated complications.
Pancreas Organ Donors
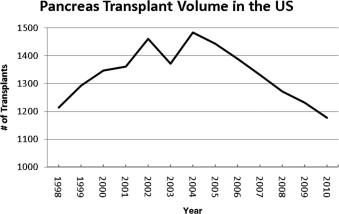
The number of pancreas transplants peaked at 1484 in 2004. Since then the volume of pancreas transplants has been decreasing ( Fig. 1 ). The vast majority of pancreas transplants originate from brain-dead donors. Although some centers accept donors after cardiac death, these donors account for less than 5% of total transplants. Additionally, since 2000, approximately 20 live-donor pancreas transplants have been performed (based on Organ Procurement and Transplantation Network data as of November 1, 2011).
The ideal pancreas donor is younger than 30 years old with a body mass index (BMI) under 25. A retrospective analysis of the Scientific Registry of Transplant Recipients was performed to calculate a pancreas donor risk index. Ten donor variables and cold ischemia time were identified as risk factors for grafts loss. The donor factors most strongly associated with poor outcome were increased age, donation after cardiac death, African American race, and higher BMI. There are multiple possible explanations for the decline in overall pancreas transplant numbers, but underutilization of potential donors is a persistent problem. Approximately 20% to 25% of potential organ donors are used for pancreas transplant, but a large number of potential donors are refused for reasons that are difficult to ascertain. Use of a group of potential pancreas donors that meet the typical clinical criteria of young age and low BMI could increase pancreas transplant volume by as much as 30%.
Surgical Procedure
The pancreas allograft is typically transplanted into the right iliac fossa and receives arterial inflow from the iliac artery. Before implantation, a Y conduit of donor iliac artery is anastomosed to the superior mesenteric and splenic arteries of the graft. The venous and exocrine routes of pancreatic drainage are the two primary technical considerations during a pancreas transplant. The portal vein of the pancreatic allograft can be anastomosed to the portal vein or iliac vein (systemic venous drainage) of the recipient. The exocrine secretions can be drained into the intestine or bladder.
Portal Versus Systemic Venous Drainage
The majority of pancreatic allografts use the iliac vessels for vascular inflow and outflow. Systemic venous drainage may be associated with lower thrombosis rates. On the other hand, portal venous drainage mimics natural physiology by allowing for first-pass degradation of insulin in the liver.
Systemic venous drainage of the pancreatic allograft has been associated with increased peripheral insulin levels and theoretically lower portal insulin levels. Hyperinsulinemia is thought to be an independent risk factor for increased ischemic cardiovascular disease. Pancreatic venous drainage into the liver via the portal vein is thought to decrease circulating insulin levels and potentially offer a metabolic advantage. In fact, portal venous drainage is associated with higher cholesterol levels, and systemic venous drainage is associated with mild hyperinsulinemia and increased insulin resistance. Glucose metabolism does not seem to be affected by the route of venous drainage when comparing patients receiving SPK transplant or kidney transplant alone, thereby controlling for the effects of immunosuppression. Any risk posed by peripheral hyperinsulinemia is more than likely offset by long-term euglycemia. Overall, portal venous drainage does not provide a significant metabolic advantage over systemic venous drainage.
Bladder Versus Enteric Drainage
During surgery the head of the pancreas is placed down into the pelvis, allowing either enteric or bladder exocrine drainage. Enteric drainage allows for delivery of pancreatic secretions into the intestine and was thought to be more physiologically advantageous. Unfortunately, complications with the side-to-side duodenojejunostomy can be life-threatening. Leak from the enteric anastomosis requires surgical exploration and can necessitate allograft pancreatectomy. To mitigate some of the risk associated with enteric drainage, some groups perform Roux-en-Y duodenojejunostomy. Additionally, enteric drainage is associated with an increased rate of graft thrombosis. Undetected early acute rejection in enteric-drained grafts might explain the increased thrombosis rate. Severe complications from enteric leaks and sepsis prompted the advent of bladder drainage by Sollinger and colleagues in the 1980s. The primary advantage of bladder drainage is the ease of monitoring the pancreatic graft function. Decreased urinary amylase precedes irreversible hyperglycemia in association with allograft acute cellular rejection. In addition, cystoscopic evaluation of the graft duodenum and graft biopsy is possible. Unfortunately, bladder drainage is associated with multiple genitourinary symptoms such as recurrent urinary tract infection, prostatitis, urethritis, and hematuria. Graft duodenal complications include bleeding and ulceration. Bladder drainage can result in severe metabolic acidosis and dehydration due to urinary bicarbonate loss. Because of these specific complications, conversion to enteric drainage is required in 30% of patients within 5 years and 50% of patients within 15 years. However, a prospective study showed that the route of exocrine drainage does not impact overall patient survival or pancreas graft function. Therefore, the majority of SPK transplant patients have enteric exocrine drainage. PA patients have a higher rate of immunologic graft loss and therefore require close surveillance. This group of patients should be considered for bladder drainage to allow for cystoscopy and biopsy.
Surgical Procedure
The pancreas allograft is typically transplanted into the right iliac fossa and receives arterial inflow from the iliac artery. Before implantation, a Y conduit of donor iliac artery is anastomosed to the superior mesenteric and splenic arteries of the graft. The venous and exocrine routes of pancreatic drainage are the two primary technical considerations during a pancreas transplant. The portal vein of the pancreatic allograft can be anastomosed to the portal vein or iliac vein (systemic venous drainage) of the recipient. The exocrine secretions can be drained into the intestine or bladder.
Portal Versus Systemic Venous Drainage
The majority of pancreatic allografts use the iliac vessels for vascular inflow and outflow. Systemic venous drainage may be associated with lower thrombosis rates. On the other hand, portal venous drainage mimics natural physiology by allowing for first-pass degradation of insulin in the liver.
Systemic venous drainage of the pancreatic allograft has been associated with increased peripheral insulin levels and theoretically lower portal insulin levels. Hyperinsulinemia is thought to be an independent risk factor for increased ischemic cardiovascular disease. Pancreatic venous drainage into the liver via the portal vein is thought to decrease circulating insulin levels and potentially offer a metabolic advantage. In fact, portal venous drainage is associated with higher cholesterol levels, and systemic venous drainage is associated with mild hyperinsulinemia and increased insulin resistance. Glucose metabolism does not seem to be affected by the route of venous drainage when comparing patients receiving SPK transplant or kidney transplant alone, thereby controlling for the effects of immunosuppression. Any risk posed by peripheral hyperinsulinemia is more than likely offset by long-term euglycemia. Overall, portal venous drainage does not provide a significant metabolic advantage over systemic venous drainage.
Bladder Versus Enteric Drainage
During surgery the head of the pancreas is placed down into the pelvis, allowing either enteric or bladder exocrine drainage. Enteric drainage allows for delivery of pancreatic secretions into the intestine and was thought to be more physiologically advantageous. Unfortunately, complications with the side-to-side duodenojejunostomy can be life-threatening. Leak from the enteric anastomosis requires surgical exploration and can necessitate allograft pancreatectomy. To mitigate some of the risk associated with enteric drainage, some groups perform Roux-en-Y duodenojejunostomy. Additionally, enteric drainage is associated with an increased rate of graft thrombosis. Undetected early acute rejection in enteric-drained grafts might explain the increased thrombosis rate. Severe complications from enteric leaks and sepsis prompted the advent of bladder drainage by Sollinger and colleagues in the 1980s. The primary advantage of bladder drainage is the ease of monitoring the pancreatic graft function. Decreased urinary amylase precedes irreversible hyperglycemia in association with allograft acute cellular rejection. In addition, cystoscopic evaluation of the graft duodenum and graft biopsy is possible. Unfortunately, bladder drainage is associated with multiple genitourinary symptoms such as recurrent urinary tract infection, prostatitis, urethritis, and hematuria. Graft duodenal complications include bleeding and ulceration. Bladder drainage can result in severe metabolic acidosis and dehydration due to urinary bicarbonate loss. Because of these specific complications, conversion to enteric drainage is required in 30% of patients within 5 years and 50% of patients within 15 years. However, a prospective study showed that the route of exocrine drainage does not impact overall patient survival or pancreas graft function. Therefore, the majority of SPK transplant patients have enteric exocrine drainage. PA patients have a higher rate of immunologic graft loss and therefore require close surveillance. This group of patients should be considered for bladder drainage to allow for cystoscopy and biopsy.
Surgical Complications After Pancreas Transplantation
Pancreas transplantation is complicated by a high rate of early technical failure. In the 1980s, worldwide technical failure rates were approximately 25%. In the subsequent 30 years the rate has fallen to approximately 7% to 9%. Surgical complications require exploration in approximately 30% of patients and almost invariably are associated with graft loss, patient morbidity, and increased cost. To a large degree, the high rate of technical failure has inhibited wider adoption of pancreas transplantation as the preferred modality for treating diabetes.
Graft thrombosis is the most frequent serious surgical complication after pancreas transplant. The symptoms of thrombosis include unexplained hyperglycemia, tenderness over the graft, graft enlargement, and in bladder-drained grafts, hematuria and decreased urinary amylase. Donor selection is critical in reducing the risk of thrombosis. The donor factors associated with thrombosis include increased donor age, massive donor volume resuscitation, hemodynamic instability, and cerebrovascular accident as cause of donor death. Poor surgical technique during organ recovery or back table preparation also increases the surgical complication rate. The use of histidine-tryptophan-ketoglutarate preservation solution also seems to increase the rate of surgical complications, particularly if the cold ischemia time exceeds 12 hours. The diagnosis of graft thrombosis is made on ultrasound, computed tomography (CT) angiography, magnetic resonance imaging, or conventional angiography. Once thrombosis is diagnosed, prompt surgical exploration is prudent to prevent leak and sepsis and to reduce the potential for mortality. Nonoperative management of thrombosis is considered in select cases, but careful patient selection is mandatory.
Early posttransplant graft pancreatitis can be difficult to definitively diagnose because hyperamylasemia is commonly seen in the early posttransplant period. Risk factors for pancreatitis include advanced donor age, donor obesity, prolonged cold ischemia time, and bladder drainage. In patients with bladder drainage, reflux pancreatitis can be treated with Foley catheter drainage. Complications of graft pancreatitis are similar to those of native pancreatitis and include peripancreatic abscess, pancreatic necrosis (sterile or infected), pancreatic fistula, and pseudocyst. Late-graft pancreatitis occurs an average of 28 months after transplant. These patients typically present with fever and graft tenderness. Late-graft pancreatitis can be adequately treated with bowel rest, antibiotics, and percutaneous drainage as needed.
Duodenal leaks from bladder-drained pancreas grafts typically occur within the first 3 months, and are easily diagnosed with a cystogram. Prolonged Foley catheter drainage is adequate treatment in up to two-thirds of cases. In complicated cases, surgical exploration and repair or enteric conversion may be required. In contrast, leakage from enteric-drained pancreas grafts frequently results in sepsis and peritonitis secondary to spillage of enteric contents. Early enteric leaks are technical, whereas late leaks are typically due to rejection, infection, or ischemia. Diagnosis of enteric leak is often made on CT scan. Prompt reexploration with conversion to Roux-en-Y drainage or even graft pancreatectomy in severely contaminated cases is required.
Intraabdominal bleeding in the early period after pancreas transplant is typically associated with systemic anticoagulation initiated to prevent graft thrombosis. It is important to rule out venous graft thrombosis of either the pancreas or kidney. Therapy is then directed toward correcting coagulation abnormalities. If a significant perigraft hematoma develops, strong consideration should be given to surgical exploration and evacuation to prevent subsequent infection of the hematoma. Gastrointestinal bleeding within 7 days of enteric-drained pancreas grafts is usually from the anastomotic suture line. This bleeding may require surgical exploration and direct control of bleeding, revision of the duodenojejunal anastomosis, or graft pancreatectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






