Pancreatic cystic lesions are being detected with increasing frequency, at least partly because of the increased use of cross-sectional imaging for the evaluation of abdominal complaints or screening for other conditions. Image-based studies report prevalences of pancreatic cystic lesions ranging from 1.2% to 19.6%. In a study of 1064 pancreatic cystic neoplasm (PCN) patients pathologically confirmed over a 12.5-year period, 108 (10.5%) of the patients had pancreatic lesions that were detected while undergoing workup for other diseases.
PCNs are reported to account for up to 60% of all pancreatic cystic lesions, followed by injury-related and inflammation-related cysts (30%). PCNs include intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), serous cystic neoplasm (SCN), solid-pseudopapillary neoplasm (SPN), cystic neuroendocrine neoplasm, ductal adenocarcinoma with cystic degeneration, and acinar-cell cystic neoplasm. The proportion of PCNs varies with population. In the Western Hemisphere, SCNs account for 32% to 39%, MCNs for 10% to 45%, IPMNs for 21% to 33%, and SPNs for less than 10% of all PCNs. A nationwide survey from Korea reports the proportions of PCN which are composed of IPMNs (41.0%), MCNs (25.2%), SPNs (18.3%), SCNs (15.2%), and others (0.3%).
The Major Pancreatic Cystic Neoplasms
The 4 major types of PCNs are IPMN, MCN, SCN, and SPN. Distinguishing among the 4 most common types of cysts is important, since the management varies with each type of cyst.
Intraductal Papillary Mucinous Neoplasms
IPMNs are defined as intraductal grossly visible (typically ≥1.0 cm) epithelial neoplasms of mucin-producing cells, arising in the main pancreatic duct or its branches. The first report was 4 cases with the triad of mucus secretion, main pancreatic duct dilatation, and a swollen duodenal papilla. Since then, the number of IPMN cases reported has been increasing significantly. This may be due to a true increase in the incidence, improvement in the understanding of IPMN, and/or increased use of cross-sectional imaging in clinical practice.
IPMNs are usually diagnosed in the elderly, often diagnosed in the seventh decade of life. There is a slight male preponderance. Although the true incidence of IPMN is unknown, IPMNs are reported to be the most common PCN. IPMN accounts for approximately 1% to 3% of pancreatic exocrine neoplasms and 20% to 50% of all PCNs. Most patients diagnosed with IPMN are asymptomatic and are usually incidentally diagnosed. Symptomatic patients present with abdominal pain, pancreatitis, weight loss, diabetes mellitus, and jaundice.
The World Health Organization (WHO) classification of tumors of the pancreas, including IPMN, was modified in 2010. In the 2000 WHO classification, IPMNs were separated according to the degree of dysplasia into intraductal papillary mucinous adenoma; intraductal papillary mucinous neoplasm with moderate dysplasia; intraductal papillary mucinous carcinoma, noninvasive; and intraductal papillary mucinous carcinoma, invasive. In the 2010 WHO classification, IPMNs were classified into IPMN with low- or intermediate-grade dysplasia (which includes the former intraductal papillary mucinous adenoma and intraductal papillary mucinous neoplasm with moderate dysplasia), IPMN with high-grade dysplasia (which is equivalent to the intraductal papillary mucinous carcinoma, noninvasive), and IPMN with an associated invasive carcinoma (changed from intraductal papillary mucinous carcinoma, invasive).
Macroscopically, depending on the pancreatic ductal system involved, IPMNs are classified as either main-duct IPMN (MD-IPMN), branch-duct (BD-IPMN), or combined-type IPMN. The clinicopathologic behavior of combined-type IPMN is similar to that of MD-IPMN. Intraductal proliferation of columnar mucin-producing cells is the main histologic characteristics of IPMN. The neoplastic epithelium may show diverse architecture and cytology. Four subtypes of IPMNs have been characterized: gastric, intestinal, pancreatobiliary, and oncocytic. In a recent report, the 4 subtypes of IPMNs were associated with significant differences in survival. Patients with gastric-type IPMN had the best prognosis, whereas those with pancreatobiliary type had the worst prognosis.
Most IPMNs are diagnosed incidentally. Even symptomatic patients present with nonspecific symptoms such as abdominal pain, malaise, nausea/vomiting, and weight loss. In patients with an associated invasive carcinoma, symptoms and signs of pancreatic ductal adenocarcinoma such as weight loss, diabetes mellitus, and/or painless jaundice may be observed.
Routine blood tests, such as complete blood count, liver function test, amylase, and lipase, are usually within normal limits or show nonspecific changes. Serum CA 19-9 and carcinoembryonic antigen (CEA) are generally not of diagnostic value. However, there is a report that serum CA 19-9 was elevated in 74% of patients with invasive IPMN and in 14% of patients with noninvasive IPMN. Furthermore, it is reported that 80% of patients with invasive IPMN had elevated serum levels of CA 19-9 and/or CEA, compared with 18% of patients with noninvasive IPMN.
Endoscopic retrograde cholangiopancreatography (ERCP) was the standard diagnostic tool for IPMN in the past. In MD-IPMN, the hallmark finding is a diffusely dilated main pancreatic duct with filling defects correlating to mucinous filling or papillary tumors.
For BD-IPMN, the affected branch ducts are cystically dilated and communicate with the main pancreatic duct. In some occasions, the cystic side branch ducts do not fill with contrast due to mucus plugging. In some cases, duodenoscopy during ERCP reveals a patulous duodenal papilla and mucin extrusion through the orifice.
The use of ERCP for the diagnosis of IPMN is limited by its invasiveness and risk of complications. In some cases, visualization of the entire pancreatic duct system is not possible because of copious amount of mucin. However, ERCP offers the advantages of cytologic sampling and intervention. In addition, it may serve as a platform for developing endoscopic technologies.
Endoscopic ultrasonography (EUS) is being increasingly used to differentiate the types of IPMNs ( Fig. 1 ). EUS demonstrates a detailed morphologic analysis of pancreatic cystic lesions, guides fine-needle aspiration (FNA), and provides fluid for subsequent cyst fluid analysis such as cytology, CEA, and DNA analysis. Furthermore, EUS may play a role in the treatment of IPMN, such as the intracystic injection of ethanol or ethanol/paclitaxel for cyst ablation. EUS findings associated with malignancy in IPMN patients include marked dilatation of the main pancreatic duct (≥10 mm) in MD-IPMN and large tumors (>40 mm) with irregular septa in BD-IPMN; mural nodule greater than 10 mm in height was associated with malignancy in both MD-IPMN and BD-IPMN. The drawbacks of EUS include operator dependence and the inability to differentiate between malignancy and areas of focal inflammation that infiltrate pancreatic parenchyma and mimic malignancy. Cyst fluid analysis often demonstrates a high concentration of CEA, reflecting the presence of a mucinous epithelium. A cut-off CEA level of 192 ng/mL has the sensitivity of 73%, specificity of 84%, and accuracy of 79% for differentiating mucinous from nonmucinous pancreatic cystic lesions. However, cyst fluid CEA differentiates neither malignant mucinous cysts from benign mucinous cysts nor malignant IPMNs from benign IPMNs. Cyst fluid cytology is rarely sufficiently diagnostic to distinguish IPMN from MCN; the result is a generic cytology report of a “mucinous cyst.” The reported sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EUS-FNA for malignant IPMN were 75%, 91%, 79%, 89%, and 86%.
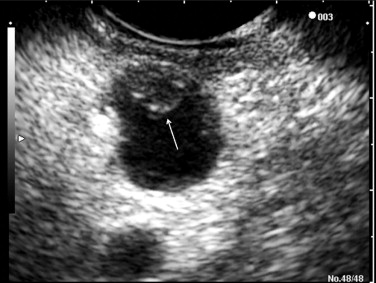
Computed tomography (CT) and magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) are used to describe the anatomic location of the IPMNs, the relationship between the lesion with surrounding organs and vessels, and the presence of distant metastasis ( Fig. 2 ). MD-IPMNs show diffuse or focal involvement of the main pancreatic duct. BD-IPMNs appear as either a cyst or a cluster of cysts, usually located in the head, particularly in the uncinate process. BD-IPMNs may be multifocal. In a report of 145 resected cases of BD-IPMN, multifocality was observed in 14.5% of patients. In another report of 190 patients with radiologically and/or histologically diagnosed BD-IPMN, 27.4% of the patients had multifocal disease. MRCP has been reported to be superior to CT in detecting ductal communication in BD-IPMN. However, with advances in multidetector CT, imaging details of CT including visualization of ductal communication have improved similar to those of MRI/MRCP.
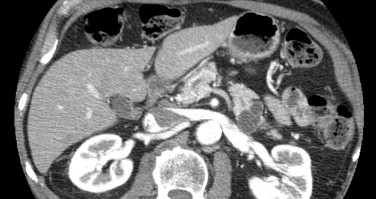
Surgical resection is often used to manage advanced IPMNs. For BD-IPMN, resection is advocated if one or more of the following criteria are met: (1) greater than 30 mm in size; (2) mural nodule(s); (3) dilated main pancreatic duct; (4) malignant pancreatic juice cytology; and (5) symptoms associated with pancreatic cysts. For resected IPMNs, the prognosis is mainly determined by the presence of an associated invasive carcinoma. For patients with resected noninvasive IPMN, the 5-year survival rate is 90% to 95%. In contrast, for patients with IPMNs with an associated invasive carcinoma, the 5-year survival rates are reported to be between 36% and 60%.
Mucinous Cystic Neoplasms
The 2010 WHO classification of tumors defines MCN as a cyst-forming epithelial neoplasm that is usually without communication with the pancreatic duct and composed of columnar, mucin-producing epithelium with an underlying ovarian-type stroma. Similar to IPMNs, MCNs are classified according to the grade of dysplasia (ie, MCN with low- or intermediate-grade dysplasia, MCN with high-grade dysplasia, and MCN with an associated invasive carcinoma).
MCNs almost exclusively occur in women, with a peak incidence in the fifth decade. The body and the tail of the pancreas are predominantly affected. Up to one-third of MCNs are reported to harbor an invasive carcinoma. Risk factors for the presence of malignancy include large tumor size, associated mass or mural nodules, and advanced age.
Around 30% of the patients may be without symptoms or signs. Symptomatic patients may complain of abdominal pain, palpable mass, weight loss, anorexia, fatigue, or jaundice. Some patients may present with pancreatitis. The results of routine laboratory testing are usually nonspecific. Patients with bile duct obstruction display a cholestatic liver function abnormality.
Macroscopically, MCNs present as single spherical masses. The lesions may be unilocular or multilocular. The cysts contain thick mucin or a mixture of mucin and hemorrhagic-necrotic material. There is no communication between the tumor and the pancreatic duct, unless there is fistula formation. The frequency of the lesion communicating with the pancreatic duct system may be high. In a recent Japanese multi-institutional report, 18.1% (25 of 138 patients) of MCNs demonstrated communication with the pancreatic duct.
Histologically, MCNs comprise 2 distinctive components: an epithelial lining and an ovarian-type stroma. Tall mucinous columnar cells line the epithelium. The ovarian-type stroma, which consists of densely packed spindle-shaped cells with round or oval nuclei, is a distinctive finding in MCN. The ovarian-type stroma is considered a requisite for the diagnosis of MCN.
On CT, MCNs appear as large cysts with thin septae; the septae are best shown after the administration of intravenous contrast. Calcifications may be seen, which are lamellated and located on the periphery of the lesion, in contrast to the central, stellate calcifications of the SCN. On MRI, the cysts have high signal intensity (bright) on T2-weighted images. On T1-weighted images with intravenous gadolinium administration, the wall and the septae are more conspicuously demonstrated.
EUS findings of MCN are thin-walled, septated fluid-filled cavities with diameter greater than 1 to 2 cm. Duct communication is rarely seen. Increased size, cyst-wall irregularity and thickening, intracystic solid regions, or an adjacent solid mass are findings suggestive of malignancy. Cyst CEA levels are high as a result of secretion by the mucinous epithelium. As mentioned, it is difficult to distinguish MCN from IPMN on the basis of cyst fluid cytology. Since MCNs rarely communicate with the pancreatic duct, ERCP is not routinely performed in the evaluation of MCNs.
Current consensus guideline advocates that all MCNs should be resected, unless there are contraindications for operation. Surgical resection is curative in nearly all patients with noninvasive MCN. For MCNs with an associated invasive carcinoma, prognosis depends on the extent of the invasive component, tumor stage, and resectability. The 2-year survival rate and 5-year survival rate of patients with resected MCN with an associated invasive carcinoma are about 67% and 50%, respectively.
Serous Cystic Neoplasms
SCNs are cystic neoplasms composed of cuboidal, glycogen-rich epithelial cells. The lesions are filled with serous fluid. According to the degree of dysplasia, they are classified as either serous cystadenoma or serous cystadenocarcinoma.
SCNs occur more frequently in women. Patients are usually diagnosed with SCN in their late 50s or early 60s. They occur more frequently in the body or the tail of the pancreas. Most patients are without symptoms or signs on diagnosis. Symptomatic patients may present with abdominal pain, palpable mass, anorexia, jaundice, fatigue/malaise, or weight loss. Nearly 90% of von Hippel–Lindau (VHL) syndrome patients are reported to develop SCNs. SCNs are rarely malignant; only about 25 malignant cases have been reported to this date.
SCNs are usually single, round lesions, with diameters that can be greater than 20 cm. On cross-section, the cysts are composed of numerous microcysts filled with serous fluid. SCNs do not communicate with the pancreatic duct. A dense fibronodular scar is often located in the center of the lesion. A single layer of cuboidal epithelial cells lines the cysts. The central scar is composed of acellular hyalinized tissue and a few clusters of tiny cysts.
Four variants of serous cystadenoma are known. The serous epithelial components of these variants are identical to those of serous cystadenoma. They are macrocystic serous cystadenoma, solid serous adenoma, VHL-associated SCN, and mixed serous neuroendocrine neoplasm. Macrocystic serous cystadenomas include previous serous oligocystic and ill-demarcated serous adenoma. Solid serous adenomas are well-circumscribed neoplasms that have a solid gross appearance; they share the cytologic and immunohistologic features of classic SCN. VHL-associated SCN describes multiple serous cystadenomas and macrocystic variants that occur in VHL syndrome patients. In patients with VHL, SCNs typically involve the pancreas diffusely or in a patchy fashion. The mixed serous neuroendocrine neoplasm is the rare entity of serous cystadenomas associated with pancreatic neuroendocrine neoplasms. This is highly suggestive of VHL syndrome.
On CT, SCNs may have the classic microcystic appearance or the less common oligocystic appearance ( Fig. 3 ). Microcystic-type lesions comprise multiple small cysts. A central fibrous scar with calcification, which occurs up to 30% in SCNs, is considered pathognomonic. The dense tissue is arranged in a stellate form. In some cases, the small cysts and dense fibrous component may make the lesions appear solid on CT.
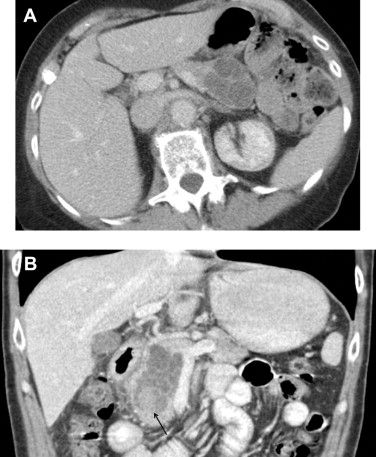
The oligocystic pattern is often difficult to differentiate from MCN on CT. Oligocystic SCNs should be suspected when a unilocular cystic lesion with lobulated contour without wall enhancement is located in the pancreatic head. On T1-weighted fat-suppressed MRI, the fluid component shows lower signal intensity compared to the fibrous matrix. On T2-weighted images, the fluid becomes bright.
On EUS, the typical SCN has multiple small, anechoic cystic areas and thin septations. Because of the vascular nature of the SCN, aspirants from EUS-FNA may be bloody or contain hemosiderin-laden macrophages. Aspirated cyst fluid is low in CEA concentration. The yield of cytology with EUS-FNA is poor.
The prognosis for patients with SCN is excellent. Even in the rare cases of serous cystadenocarcinoma, there are reports of a long-term survival after resection. Currently, proposed indications for surgical resection are presence of symptoms, size of greater than 4 cm, and uncertainty about the nature of the cystic neoplasm. Although increased size does not predict malignancy, large SCNs are reported to grow at a faster rate and are more likely to cause symptoms.
Solid-pseudopapillary Neoplasms
SPNs are low-grade malignant neoplasms composed of monomorphic epithelial cells that form solid and pseudopapillary structures. SPNs frequently undergo hemorrhagic-cystic degeneration.
SPNs occur predominantly in young women. The mean age at diagnosis is in the patient’s 20s or 30s. Symptomatic patients may present with pain, mass, anorexia, nausea/vomiting, jaundice, or weight loss. SPNs are reported to occur evenly throughout the pancreas.
Macroscopically, SPNs are large, round, single masses (average size, 8–10 cm). They are well demarcated and often fluctuant. The cut section discloses lobulated solid areas and zones with a mixture of hemorrhage, necrosis, and cystic degeneration. Microscopically, they are a combination of solid pseudopapillary component and hemorrhagic-necrotic pseudocystic components. The solid portion is formed with poorly cohesive monomorphic cells and myxoid stromal bands containing thin-walled blood vessels. When the poorly cohesive neoplastic cells fall out, the remaining neoplastic cells and the stroma form the pseudopapillae. The neoplastic cells have eosinophilic or clear vacuolated cytoplasms. Mucin is absent, and glycogen is not conspicuous. SPNs without histologic criteria of malignant behavior, such as perineural invasion, angioinvasion, or infiltration of the surrounding parenchyma, may metastasize. Therefore, all SPNs are classified as low-grade malignant neoplasms.
On CT, SPNs appear as well-circumscribed, encapsulated masses with varying areas of soft tissue and necrotic foci. The capsule is usually thick and enhancing. Peripheral calcification has been reported up to 30% of patients. No septations are visualized. On MRI, the neoplasm is shown as a well-defined lesion with a mix of high and low signal intensity on T1- and T2-weighted images, which reflects the complex nature of the mass. Areas filled with blood products demonstrate high signal intensity on T1-weighted images and low or inhomogeneous signal intensity on T2-weighted images.
On EUS, SPNs are usually well-defined, hypoechoic masses. They may be solid, mixed solid and cystic, or cystic. Internal calcifications can be seen in some patients. The reported diagnostic accuracy of EUS-FNA for SPN based on cytology and immunohistochemistry is 65%. Aspirated cyst fluid may display necrotic debris. The cyst fluid CEA is low, reflecting the presence of nonmucinous epithelium.
The mainstay of treatment is surgery. After complete surgical resection, 85% to 95% of patients are cured. Even in cases with local invasion, recurrences, or metastases, long-term survival have been documented. No definite biological or morphologic predictors of outcome have been documented. Suggested indicators of poor outcome include old age and SPNs with an aneuploidy DNA content.
Recent Developments
Recent developments in diagnosis of PCNs have been made in the analysis of cystic fluid and high-resolution imaging. For the treatment of PCNs, EUS-guided cyst ablation has shown promising results.
Cyst Fluid Analysis
DNA analysis of pancreatic cyst fluid demonstrated that KRAS mutation is highly specific (96%) for mucinous cysts. Elevated amounts of cyst fluid DNA, high-amplitude mutations, and specific mutation sequences were indicators of malignancy. High-amplitude KRAS mutation followed by allelic loss was the most specific marker for malignancy. Cyst fluid interleukin-1β concentration has been shown to be higher in malignant IPMN than benign IPMN.
High-resolution Imaging
Ex vivo optical coherence tomography (OCT) of freshly resected pancreatectomy specimens demonstrated that mucinous cysts could be differentiated from nonmucinous cysts with high sensitivity (>95%), specificity (>95%), and almost perfect interobserver agreement. OCT is an interferometric technique that typically uses near-infrared light. It allows noninvasive micron-scale cross-sectional imaging of biological tissues by measuring their optical reflections.
Confocal laser endomicroscopy (CLE) is a novel imaging technology that uses low-power laser to obtain in vivo histology of the gastrointestinal mucosa. Needle-based CLE (nCLE) enables the performance of CLE in intra-abdominal organs under EUS guidance or via natural-orifice transluminal endoscopic surgery procedures. Konda and colleagues reported the feasibility of nCLE during EUS-FNA of pancreatic lesions in 18 patients with pancreatic lesions (16 cysts and 2 masses). The nCLE miniprobe was introduced into the lesion through a 19-gauge FNA needle. Technical feasibility was achieved in 17 cases. Image quality was good to very good in 10 cases. Two serious adverse events of pancreatitis requiring hospitalization were reported.
Pancreatic Cyst Ablation
The introduction of curvilinear EUS and EUS-FNA made EUS-guided injection therapy possible. EUS-guided pancreatic cyst ablation is achieved by the injection of a cytotoxic agent after puncture of the pancreatic cyst under EUS guidance. Injection of a cytotoxic agent may result in ablation of the cyst epithelium.
The first cytotoxic agent used was ethanol. In the initial study, various concentrations of ethanol (5%–80%) were injected into the pancreatic cysts of 25 patients. There were no reported complications. Eight patients had complete resolution of pancreatic cysts. Histologic evidence of epithelial ablation was seen in 5 patients who underwent resection. A prospective randomized multicenter trial comparing lavage of pancreatic cysts with 80% ethanol to lavage with saline was conducted in 2 centers. Ethanol lavage resulted in a greater mean percentage of cyst surface area decrease than saline. The overall pancreatic cyst resolution defined by CT was 33.3%. Histology of 4 resected specimens demonstrated 0% epithelial ablation in 1 saline lavage case and 50% to 100% epithelial ablation in 3 ethanol lavage cases. Complication rates were similar in both groups, with one major complication of acute pancreatitis requiring hospitalization in the ethanol group. Long-term follow-up results of patients from this study were reported in 2010. In 9 patients, follow-up CTs performed after a median follow-up period of 26 months after initial documentation of cyst resolution demonstrated no evidence of cyst recurrence.
EUS-guided ethanol lavage with paclitaxel injection (EUS-ELPI) for pancreatic cysts was recently introduced in Korea. This technique involves the aspiration of cyst fluid under EUS guidance, lavaging the cyst with 99% ethanol, re-aspiration of ethanol, and injecting paclitaxel (concentration of 3 mg/mL) into the cyst. The volume of the injected paclitaxel is the same as the volume of the aspirated cyst fluid. A study involving 52 patients who underwent EUS-ELPI for pancreatic cysts was reported in 2011. Forty-three patients were followed for longer than 12 months, and 4 patients underwent surgery for persistent cysts; 5 patients were excluded from analysis. A complete response was observed in 29 patients, partial response in 6, and persistent cyst in 12. For 4 patients who underwent resection, histopathology revealed variable epithelial ablation extent of 0%, 25%, 40%, and 100%. Small cyst volume was the only independent factor associated with complete cyst resolution. Complications reported were fever without bacteremia (n = 1), abdominal discomfort of 2-weeks’ duration (n = 1), pancreatitis (n = 1), pericystic spillage (n = 1), and splenic vein obliteration with collateral formation (n = 1).
The early results of EUS-guided pancreatic cyst ablation are promising. However, more data on long-term follow-up and efficacy are needed to define precise indications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






