Immunofluorescence
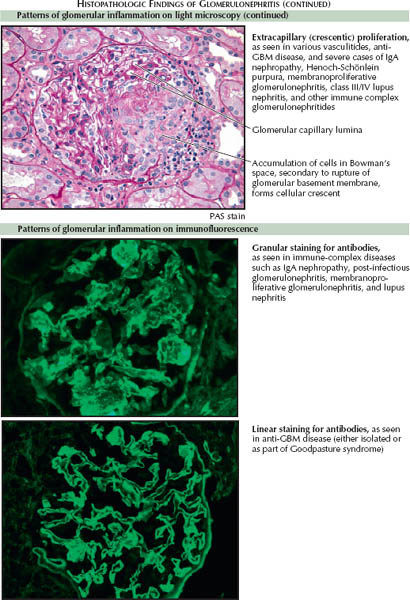
The pattern observed on immunofluorescent staining of the glomerulus for antibodies and/or complement proteins can often be described as granular, linear, or pauci-immune. These patterns offer clues into the underlying disease process.
Granular. A pattern of patchy, granular staining for antibodies and complement proteins suggests immune complex deposition. The deposited complexes fix complement, leading to direct complement-mediated damage and, frequently, endocapillary proliferation.
IgA nephropathy is the most common cause of immune complex GN and the most common cause of GN in general. Other causes of immune complex GN include lupus nephritis, membranoproliferative GN, and postinfectious GN. These diseases are typically associated with depressed complement levels, reflecting their mechanism of inflammation; however, complement levels may remain normal in IgA nephropathy because of the slow rate at which the various components are consumed.
Linear. A pattern of continuous, linear staining for antibodies and complement proteins along the capillary walls suggests direct binding of antibodies to the glomerular basement membrane (GBM). Such binding occurs in the anti-GBM diseases, in which autoantibodies form against the noncollagenous 1 (NC1) domain of the α-3 chain of type IV collagen (α-3 IV). The resulting inflammation almost invariably leads to RPGN.
In the spectrum of anti-GBM disease, one third of patients have renal-limited disease, whereas the remainder have the combined pulmonary-renal syndrome named for Goodpasture. Goodpasture syndrome occurs when the anti-GBM autoantibodies bind to the alveolar basement membrane, causing pulmonary hemorrhage. This syndrome occurs almost exclusively in smokers and others exposed to hydrocarbons, suggesting that environmental factors play a key role in determining the susceptibility of the pulmonary capillaries to circulating anti-GBM autoantibodies.
Pauci-immune. A general lack (or, as the name suggests, paucity) of staining for antibodies or complement proteins suggests vasculitic GN. In such cases, inflammation generally reflects the presence of circulating antineutrophil cytoplasmic antibodies (ANCAs), which are believed to directly activate neutrophils. The ensuing glomerular inflammation often leads to RPGN, and it may be either isolated or part of a systemic vasculitis. Other common histologic findings include fibrinoid glomerular necrosis, periglomerular inflammation, and arteritis.
ANCAs can be subdivided into those with a cytoplasmic staining pattern (c-ANCA) and those with a perinuclear staining pattern (p-ANCA). c-ANCAs almost always target proteinase-3 antigens (PR3-ANCA) and are often associated with Wegener granulomatosis. p-ANCAs almost always target myeloperoxidase antigens (MPO-ANCA) and are often associated with Churg-Strauss disease and microscopic polyangiitis. The ANCA-positive systemic vasculitides all feature a propensity toward multiple organ involvement. Specifically, Wegener granulomatosis and microscopic polyangiitis are associated with pulmonary hemorrhage, whereas Churg-Strauss features asthma and eosinophilia.
< div class='tao-gold-member'>




