Fig. 8.1
Completion of end-to-end portal vein anastomosis in orthotopic liver transplantation. The running suture was not fully tied, leaving a loose tract of the thread (“growth factor”) to allow distension of the anastomosis after reperfusion, thus preventing portal vein stricture
The sequence of vascular clamp release is variable, but usually the suprahepatic vena cava is unclamped first, followed by the portal vein and lower IVC. At this stage, the anesthesiology team must keep the central pressure low in order to avoid an excessive cardiac preload that could affect adequate venous outflow from the graft.
The arterial anastomosis is usually performed in an end-to-end fashion between the branch patch obtained between the recipient CHA and the origin of the GDA and the CHA of the donor liver. On the recipient side, the arterial stump obtained by extending the orifice of the CHA to the GDA is usually as large as possible, and it prevents any steal phenomenon through the GDA, whose distal stump is carefully sutured. At the same time, using the donor CHA normally allows a short and straight reconstruction. Performing the anastomosis on the donor celiac trunk has the theoretical advantage of a wider stump on the donor side, but it usually determines an excessive length of the post-anastomotic arterial axis, with the consequent risk of kinking. In addition, the donor celiac artery, and even more the Carrel patch, is much more frequently involved by atherosclerotic plaque than the CHA in the case of older donors. Depending on the size of the arteries, the anastomosis is usually performed with 6/0, 7/0, or 8/0 PROLENE® sutures, which can be a running or an interrupted one. Wider arterial orifices, especially on the donor side, can be obtained by creating oblique stumps.
Other recipient sites for arterial reconstruction can be the proper hepatic artery, especially at the bifurcation of the right and left branches, or an accessory/replaced right hepatic artery (aRHA) from the superior mesenteric artery (SMA) in the case of absent or small CHA, or the splenic artery (SA) close to its origin from the aorta in the case of unsuitability of any HA [7]. In this latter case, the reconstruction may require an interposition donor arterial graft due to the distance between SA and CHA, and the anastomosis can be performed in an end-to-end or an end-to-side fashion. A careful evaluation of possible consequences is necessary when using the recipient SA, because an end-to-end anastomosis requires the interruption of arterial flow to the spleen through the SA itself, while an end-to-side anastomosis could determine a steal phenomenon by the spleen.
When the recipient HA is unsuitable, as in the case of very small size or dissection due to surgical maneuvers, or previous intra-arterial treatments, arterial revascularization can also be obtained by interposing an arterial vascular graft between the infrarenal aorta, or the supraceliac aorta [8], and the HA. In such cases, a donor iliac artery is more frequently used as an interposition graft. The recipient aorta is isolated and clamped longitudinally, and an end-to-side anastomosis is performed first with the vascular graft, usually with a 5/0 or 6/0 PROLENE® running suture. If the infrarenal aorta is used, the arterial graft is then passed through the transverse mesocolon, behind the stomach, and in front of the pancreas, for the subsequent end-to-end anastomosis with the donor HA.
In around 20–30 % of cases, the liver graft presents with arterial anomalies, more frequently consisting in an aRHA (from the SMA), an accessory left hepatic artery (aLHA, from the left gastric artery), or a combination of the above.
In the case of an aRHA, it is usually reconstructed in an end-to-end fashion with the donor GDA (Fig. 8.2). Alternatively, it can be reconstructed on the stump of the donor SA. The aRHA can be preserved also by creating a single donor arterial stump with an end-to-end anastomosis between the celiac artery and the SMA (the so-called mesenteric conduit), which is subsequently anastomosed to the recipient HA. However, this technique may lead to an excessively long arterial tract.
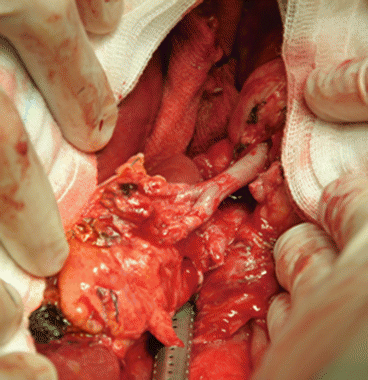

Fig. 8.2
Reconstruction of a donor accessory right hepatic artery on the stump of the donor gastroduodenal artery
In the case of an aLHA, its preservation requires a reconstruction between the recipient HA and the donor celiac artery, with the already-mentioned risk of kinking. In most cases, the aLHA is very small and can simply be ligated. If the aLHA is very large, after HA reconstruction the entire arterial axis is rechecked for its length after positioning of the aLHA deeply below the left lobe of the liver. If kinking is visible, the HA can be shortened, with a subsequent end-to-end anastomosis (Fig. 8.3).
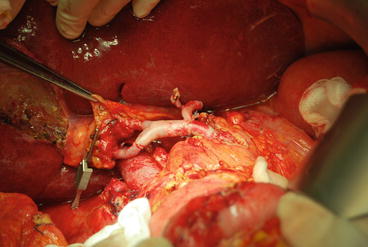

Fig. 8.3
Liver graft with a common hepatic artery and two accessory arteries (right and left). In this case, the accessory right hepatic artery was reconstructed on the donor gastroduodenal artery after anastomosis between the recipient common hepatic artery and the donor celiac artery. However, the entire arterial tract appeared too long and at risk of kinking; thus, the donor common hepatic artery was shortened with a subsequent end-to-end anastomosis
In general, when multiple reconstructions are anticipated, the arterial backflow through the stumps of accessory arteries should be checked, and an intraoperative color Doppler ultrasound examination should be performed in order to quantify the parameters of perfusion of areas vascularized by accessory arteries. If a pulsate backflow is present and the left lateral segment or the right posterior segments display a normal arterial flow pattern, the reconstruction of accessory arteries is not strictly necessary.
In our early experience, grafts from older donors, the use of interposition arterial grafts, multiple arterial reconstructions, and the absence of antiplatelet prophylaxis were predictors of arterial complications [9]. However, in a more recent experience, only interposition grafts and HA anatomical variations remained factors independently related to HA thrombosis after LT [10].
When a portal vein thrombosis is present in the recipient, different types of portal vein reconstruction have been suggested and will be discussed in a dedicated section. In these cases, a completely normal or physiological portal flow might not be restored. Thus, completion of the HA reconstruction is advisable before portal reperfusion, with subsequent simultaneous arterial and portal reperfusion. This strategy may lead to a slightly longer warm ischemia time, but it ensures adequate graft revascularization.
In general, there is no absolute agreement on the best sequence of graft reperfusion, which can be retrograde (by releasing the caval clamp first), portal, or arterial. However, initial portal vein revascularization is certainly the most frequently adopted [11, 12].
The biliary reconstruction is usually performed between the donor and recipient main bile ducts in an end-to-end fashion, using a 5/0 or 6/0 PDS® running or interrupted suture.
The bile duct stumps must be well vascularized, and in this sense electrocauterization should be avoided around these elements. Ideally, the sectioning of the donor and recipient bile ducts is best performed above and below the junction of the cystic ducts, respectively, provided that the two bile duct stumps are long enough to be anastomosed without stretching. These sectioning sites guarantee good vascularization and eliminate the risk of posttransplant dilation of a retained cystic duct, which could be progressively filled with mucus and compress the bile duct, causing its stricture.
A T-tube can be inserted into the bile duct before completion of the anastomosis of the anterior walls. The upper and lower short branches of the tube are placed across the anastomosis and in the distal bile duct, respectively, while the long branch is brought out through the recipient bile duct wall around 2 cm distally to the anastomosis and then through the abdominal wall. The T-tube has the advantage of allowing intra- and postoperative cholangiography, maintaining a patent lumen in the case of tendency to stricture for different causes (technical, vascular), and draining the bile outside in the case of bile leaks. The main disadvantage lies in the possibility of bile leaks during T-tube removal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






