Although the aging process per se can produce measurable changes in the normal oropharyngeal swallow, these changes alone are rarely sufficient to cause clinically apparent dysphagia. The causes of oropharyngeal dysphagia in the elderly are predominantly neuromyogenic, with the most common cause being stroke. The evaluation of oropharyngeal dysphagia in the elderly involves early exclusion of structural abnormalities, detection of aspiration by videofluoroscopy which might dictate early introduction of nonoral feeding, and exclusion of underlying systemic and neuromyogenic causes that have specific therapies in their own right. Such conditions include Parkinson disease, myositis, myasthenia, and thyrotoxicosis. Management is best delivered by a multidisciplinary team involving physician, speech pathologist, nutritionist and, at times, a surgeon.
The aging process is associated with measurable changes in nerve and muscle function. The commonest causes of pharyngeal dysphagia are neuromyogenic disorders. Although the aging process is associated with measurable changes in muscle function, aging does not usually cause pharyngeal dysphagia. However, the aging process is associated with an increased prevalence of neuromuscular disorders and systemic and degenerative processes that can be associated with or cause pharyngeal dysphagia. Hence, in the aging population, oropharyngeal dysphagia can frequently lead to severe malnutrition, aspiration, pneumonia, and death. It is common in the chronic care setting, with 60% to 87% of occupants in aged care facilities having feeding difficulties, of whom a substantial proportion have dysphagia. It is recognized that inability to feed oneself in such a setting leads to a much higher mortality rate. In the general community, dysphagia is probably under-recognized in the elderly. A Dutch study reported symptomatic dysphagia in 16% of individuals older than 87 years. Fibrosing disorders of the cricopharyngeus such as Zenker diverticulum, has an average age of presentation in the late ninth decade. The commonest cause of dysphagia in the elderly is stroke, and this carries a high morbidity, mortality, and cost. For example, dysphagia following stroke is an independent predictor of institutionalization or subsequent hospital readmission. Oropharyngeal dysphagia occurs in one third of all stroke patients. The incidence of conditions such as Parkinson disease and Alzheimer disease increase with aging and these disorders demonstrate a 20% to 50% prevalence of oropharyngeal dysphagia. Therapeutic response and prognosis is variable and is dependent on several factors including the underlying cause, the severity and nature of the mechanical dysfunction, and the degree of associated cognitive dysfunction when present. Management of these patients generally demands a multidisciplinary approach involving the gerontologist, radiologist, gastroenterologist, neurologist, speech-language pathologist, dietician, and, at times, the palliative-care physician.
The influence of the normal aging process on neuromuscular function and on the oropharyngeal swallow
Normal aging results in changes in nerve function, a region-dependent decline in muscle mass, and cerebral atrophy and central neuronal drop-out. Age-related alterations in cortical activation are observed during swallowing. This study found an increase in somatosensory cortical activation during swallowing in the elderly. The significance of this is unknown and the findings are likely to be nonspecific with an age-related increase in cortical excitability. Diffuse periventricular white matter changes apparent on MRI are believed to be related to microvascular disease in otherwise neurologically normal aged individuals. Affected individuals, not complaining of dysphagia, show prolongation of components of the swallow that correlate to a degree with the extent of such white matter changes and, if severe enough, may account for functionally significant oropharyngeal dysphagia in some cases in which the underlying cause of dysphagia is not apparent. Concomitant medical illnesses, particularly if sufficient to require hospital admission, have been found to be associated with an increased likelihood of detecting pharyngeal dysfunction and some impairment of pharyngeal bolus clearance, even in patients who are not reporting dysphagia.
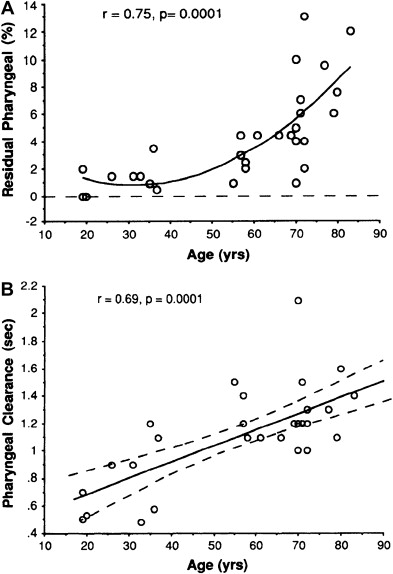
Videofluoroscopic changes in the pharyngeal swallow have been reported in up to 84% of asymptomatic elderly, compared with what is considered normal in healthy young adults. However, it must be appreciated that a mild increase in hypopharyngeal postswallow residue, or even age-related structural changes, are generally asymptomatic and do not necessarily lead to clinically apparent problems unless they are severe or unless they are associated with additional pathologic neuromyogenic dysfunction. For example, the cricopharyngeal bar is a common, frequently incidental radiological finding. This finding is demonstrated in 5% to 19% of patients undergoing pharyngeal videoradiography but is generally asymptomatic. Dysphagia is no more prevalent in those individuals found to have a cricopharyngeal bar (13%) than it is in those without a bar. Nonetheless, the overall duration of the oral phase and the pharyngeal swallow measured radiographically is prolonged, whereas the coordination among motor events measured manometrically is unaffected by normal aging. Despite these measurable differences, the pharyngeal swallow is remarkably robust in the face of the aging process, as shown by the relative preservation of bolus clearance demonstrated scintigraphically. In that study, there was no increase in bolus residue postswallow until after the age of 55 years and, even then, the pharyngeal clearance mechanism was remarkably efficient ( Fig. 1 ).
There have been several manometric studies of the effects of normal aging on the upper esophageal sphincter (UES) and pharyngeal motor function. In healthy, asymptomatic elderly patients, UES resting tone is either normal or slightly reduced and UES relaxation is complete. Peak pharyngeal contraction pressures are also preserved, or even increased, in the aged. The UES in the healthy aged does show some loss of elasticity or compliance, as manifest by some reduction in sphincter opening and resulting increase in hypopharyngeal intrabolus pressure in comparison to young controls.
Sensory nerve function is important for the normal swallow. Normal aging is associated with demonstrable changes in sensory function in the oral cavity and the pharynx. There is an increase in the threshold needed to trigger the pharyngeal swallow, and sensory discrimination of hypopharynx and the supraglottic structures is diminished in the aged. This could render the aged pharynx more liable to aspiration in the context of additional neuromuscular dysfunction.
Age-related changes in the oral cavity can be important contributors to dysphagia in the aged. The edentulous patient can have impaired masticatory function, which can be further impaired by sarcopenia and adipose tissue replacement in the tongue, and reduced masticatory strength. Aging, particularly in the context of multiple medications, leads to reduced salivary flow. Xerostomia is common, particularly in the elderly, occurring in 16% of men and 25% of women, and adversely affects swallowing by impairing swallow initiation and removing the normal lubricating function of saliva that would normally facilitate bolus transport.
Causes of oropharyngeal dysphagia in the elderly
These causes are essentially the same as for younger adults, but their prevalence generally increases with age. The etiologic classification can be broadly divided into structural or neuromyogenic causes. Neuromyogenic causes can be further subdivided into neurogenic, myogenic, metabolic, and endocrine ( Table 1 ).
| Central nervous system |
| Stroke |
| Extrapyramidal syndromes (Parkinson, Huntington chorea, Wilson disease) |
| Brainstem tumors |
| Alzheimer disease |
| Amyotrophic lateral sclerosis |
| Drugs (phenothiazines, benzodiazepines) |
| Peripheral nervous system |
| Spinal muscular atrophy |
| Guillain-Barré syndrome |
| Postpolio syndrome |
| Drugs (botulinum toxin, procainamide, cytotoxics) |
| Myogenic |
| Myasthenia gravis |
| Dermatomyositis, polymyositis, inclusion body myositis |
| Thryotoxic myopathy |
| Paraneoplastic syndromes |
| Drugs (amiodarone, alcohol, cholesterol-lowering drugs) |
| Structural disorders |
| Zenker diverticulum |
| Cricopharyngeal bar or stenosis |
| Cervical (mucosal) web |
| Oropharyngeal tumors |
| Head and neck surgery |
| Radiotherapy |
Structural Causes of Oropharyngeal Dysphagia
Tumors, head and neck surgery, radiotherapy
Tumors arising from the tongue, palate, pharynx, tonsil, and glottis may present with dysphagia. Radiology and nasoendoscopy are the major diagnostic modalities. Less commonly, extrinsic tumors of the head and neck (eg, thyroid) can also cause dysphagia if they reach a substantial size. Surgical resection of head and neck cancer commonly causes oropharyngeal dysphagia. The impact of head and neck cancer surgery on swallowing varies markedly among individuals and depends on many factors including the extent of surgical resection, whether flap reconstruction is required, which muscular, boney, or cartilaginous structures are removed or deranged, whether surgery causes collateral damage to neural innervation, and whether surgery is accompanied by radiotherapy, which damages muscles and nerves. Because tongue base motion is so important for the generation of pharyngeal propulsive forces, the extent of tongue resection is the most important factor determining dysphagia severity in those undergoing surgery for oral cancer, and the degree of preservation of tongue base motion is an important predictor of recovery of swallow function following laryngeal surgery. Laryngectomy, with or without radiotherapy, can cause dysphagia due to a combination of anatomic derangements and pharyngeal muscular dysfunction.
Radiotherapy is well recognized to cause severe nerve and muscle damage to oral and pharyngeal structures. It is not uncommon for radiation-induced dysphagia to manifest clinically more than 10 years after administration of treatment. Radiation-induced xerostomia in addition is an important contributor to swallow dysfunction in the cancer patient.
Postcricoid web, cricopharyngeal bar, and stenosis
A postcricoid web is a thin, shelflike, usually eccentric but sometimes circumferential, constriction that occurs in the proximal few centimeters of the esophagus and is comprised of a thin layer of mucosa and submucosa. Webs typically present with dysphagia for solids but because of their proximal location, deglutitive aspiration may occur. One consecutive series of 1134 videofluoroscopic examinations reported a cervical esophageal web in 7.5% of unselected patients investigated for dysphagia, and were twice as common in women as in men. Webs are frequently more readily appreciated on barium swallow than they are endoscopically. Inadvertent disruption of the lesion at endoscopy and a somewhat retrospective appreciation of its existence is common.
A cricopharyngeal bar is a common incidental radiological finding (see earlier discussion). Although frequently asymptomatic, it can cause function impairment to the swallow if it is associated with a Zenker diverticulum, if it is tightly stenosed, or if there is coexistent neuromyogenic disease ( Fig. 2 ). Compared with appropriate disease controls without myopathy, the prevalence of the postcricoid bar is significantly more common than expected in the context of inflammatory myopathies.
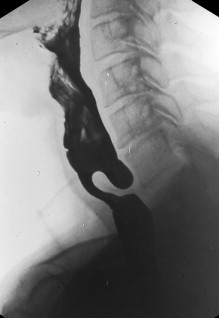
These structural lesions, when considered to be responsible for the patient’s symptoms, are treated by mechanical disruption, either by cricopharyngeal myotomy or dilatation. Myotomy is most efficacious when applied to patients with structural disorders that limit opening of the cricopharyngeus in association with preserved pharyngeal contractility as seen in webs and stenoses. Evidence supporting efficacy of either dilatation or myotomy for cervical esophageal webs and postcricoid stenosis is all uncontrolled, but consistently favorable. However, repeated dilations over many years seem to be required in at least 40% of such patients and, in one study, 20% eventually required surgery.
Zenker diverticulum
The posterior hypopharyngeal pouch, Zenker diverticulum, arises in the posterior hypopharyngeal wall through an area of relative muscular weakness (Killian dehiscence) just proximal to the upper margin of the cricopharyngeus muscle ( Fig. 2 ). The diverticulum develops secondary to fibrosis and loss of compliance of the cricopharyngeus muscle with concomitant swallow-induced increase in intrabolus flow pressures just proximal to the sphincter. Presenting symptoms typically include dysphagia and regurgitation. Aspiration symptoms and recurrent chest infections are common.
Cricopharyngeal myotomy, either alone or in combination with pouch resection or suspension, is the treatment of choice. Myotomy can be performed either by a transcutaneous or an endoluminal approach. Resection of the pouch alone is inadequate treatment and myotomy is the essential element in treatment of this condition. Simple cricopharyngeal dilatation can afford symptomatic benefit of variable duration and is a reasonable alternative in the elderly with significant comorbidity. No controlled trials of the efficacy of surgical treatment of Zenker diverticulum exist. However, the consistency of published response rates of 80% to 100% is in keeping with the strong clinical impression that surgery is nearly always curative in this disorder.
Neurogenic Causes of Oropharyngeal Dysphagia
Stroke
Stroke is the commonest cause of oropharyngeal dysphagia. Although much more common and more severe in bilateral or brainstem stroke, dysphagia affects 25% to 40% of patients in the acute phase of a unilateral hemisphere stroke. Recent studies on the cortical topographic representation of swallow musculature in health and following hemispheric stroke have implicated hemispheric asymmetry as a determinant of dysphagia following unilateral hemisphere stroke. Whether or not an individual develops dysphagia following stroke seems to be determined by the size of pharyngeal representation within the affected cortex. The degree of natural recovery of swallowing following stroke parallels the increase in size of cortical representation in the intact hemisphere. Hence, recovery of swallow function after stroke depends on the presence of intact projections from the undamaged hemisphere which, by the process of “plasticity,” can develop increased control over the brainstem swallow center with time.
Of stroke patients with dysphagia, 45% to 68% are dead within 6 months largely due to dysphagia-related nutritional and pulmonary complications. In addition to a higher mortality, dysphagia confers a higher risk of infection, poor nutrition, longer hospital stay, and institutionalization. The most prevalent complication of stroke-related pharyngeal dysphagia is aspiration pneumonia, occurring in one third of all patients and in two thirds of those with brainstem stroke. Hence, determination of the risk of aspiration in this population is a fundamental aim of management. Bedside evaluation underestimates the prevalence of deglutitive aspiration. Videofluoroscopy is vital in the assessment of aspiration risk because it can detect aspiration not evident at the time of bedside assessment in 42% to 60% of patients. This may represent a laryngopharyngeal sensory deficit as regional mucosal sensory thresholds have been found to be increased in stroke cases, compared with controls. If aspiration of all trialed consistencies is demonstrated, immediate introduction of nonoral feeding is indicated. Additional typical videofluoroscopic findings might include difficulty in initiating the swallow, a delayed or absent pharyngeal swallow response, pharyngeal weakness with poor pharyngeal clearance, and postswallow pooling in vallecula and pyriform sinuses. Does systematic evaluation of the stroke patient with dysphagia reduce the risk of pneumonia and influence outcome? There are no randomized controlled studies that address this question. Although the level of evidence is weak, reports of pneumonia rates from centers with dysphagia programs compared with historical data from centers without formal programs suggest that systematic evaluation by videofluoroscopy or nasoendoscopy coupled with a structured treatment program may reduce pneumonia rates.
Parkinson disease
Dysphagia occurs commonly in Parkinson disease and in related parkinsonian disorders such as dementia with Lewy bodies, corticobasal degeneration, multiple system atrophy, and progressive supranuclear palsy. The median survival time from onset of dysphagia to death in these related disorders is short, ranging from 15 to 24 months. The true prevalence of dysphagia in Parkinson disease is uncertain but may be as high as 52%. Drooling of saliva is even more common than dysphagia, being reported in up to 78% of patients. Although neither disease duration, nor severity, nor specific cardinal parkinsonian features correlate with the severity of dysphagia, the latency from disease onset to onset of dysphagia correlates positively with long-term survival.
Impaired preparatory lingual movements and mastication, piecemeal swallows, increased oral residue, preswallow spill, and swallow hesitancy are common radiological observations. Lingual tremor seems to be specific for extrapyramidal movement disorders. Intra- or postswallow aspiration occurs in one third of those with dysphagia, and silent aspiration has been reported in up to 15% of those reporting neither dysphagia nor symptoms of aspiration.
Manometric studies may demonstrate diminished pharyngeal contraction pressures, pharyngo-sphincteric incoordination, or synchronous pressure waves, and failure of UES relaxation is common, occurring in up to 25% of cases. Cricopharyngeal myotomy would seem to be a logical treatment in view of the high prevalence of failed UES relaxation. A favorable response to myotomy has been reported in a small series, many of whom had additional structural abnormalities, but more data are required before surgery can be recommended for the condition. Unfortunately, levodopa therapy is frequently disappointing in terms of lack of favorable changes in pharyngeal mechanics dysphagia severity. Nevertheless, evidence for a good clinical response to drug therapy exists in isolated case reports, and optimal pharmacotherapy generally improves the patients’ ability to feed themselves by minimizing hand tremor and bradykinesia, and treatment of mood disturbance, when present, may also improve feeding behavior and appetite. Appropriate timing of medication, 1 hour before meals, would seem logical and was found to be beneficial in at least one case report. Systematic reviews of therapeutic outcome have concluded that there are no controlled data on which to base firm treatment guidelines in this disease. However, the general principles of swallow rehabilitation applicable following stroke are generally adopted in Parkinson disease (see later discussion).
Motor neuron disease
Oropharyngeal dysphagia affects most sufferers at some stage in their disease. Bulbar involvement with dysarthria and dysphagia is a primary manifestation in 25% to 30% of cases of amyotrophic lateral sclerosis. The onset of the disease is insidious. Early symptoms include deglutitive cough, followed by progressive dysphagia and weight loss. The sequence of involvement of bulbar muscles is predictable in that the tongue is generally involved early and nearly always before the pharyngeal muscles. Aspiration pneumonia is a common complication, which, coupled with diminished respiratory muscle reserve, is the commonest cause of death.
Videofluoroscopic findings depend on the stage of disease. By the time dysphagia is a significant problem, lingual dysfunction is almost invariably present and manifests as repetitive tongue movements, premature retrolingual bolus spill, and significant retention of barium in the oral sulci requiring several swallows for clearance. The pharyngeal swallow response may be delayed and is eventually lost with markedly impaired bolus clearance from the pharynx and intra- and postswallow aspiration of contrast. Management of these patients is difficult and necessitates involvement of the palliative care team along with institution of swallow therapy (see below), appropriately timed introduction of percutaneous endoscopic gastrostomy (PEG) feeding, and control of troublesome drooling. In addition to videofluoroscopic swallow assessments, monitoring of vital capacity and speech are useful prognostic indicators that guide the clinician as to the timing of intervention with swallow therapy and consideration of nonoral feeding. However, there are no adequately controlled studies from which clear, evidence-based guidelines can dictate the optimal timing of PEG tube insertion. Whether or not early PEG placement results in increased survival or improved outcomes has not yet been demonstrated convincingly. The decision is made on clinical grounds, considering a range of factors including nutritional and hydration status, aspiration risk, and patient attitude to dietary modification and the feeding process.
Centrally acting drugs causing oropharyngeal dysphagia
Drugs with dopamine antagonist action, such as phenothiazines and metoclopramide, can cause dystonia and dyskinesia resulting in dysphagia ( Table 2 ). These centrally acting drugs may also impair pharyngeal propulsive and clearance functions by causing a clinical picture similar to Parkinson disease. The benzodiazepines nitrazepam and clonazepam have been documented to cause oropharyngeal dysphagia.
| Centrally acting drugs: |
| Phenothiazines a |
| Metoclopramide a |
| Benzodiazepines a (nitrazepam, clonazepam) |
| Antihistamines a |
| Drugs acting at neuromuscular junction: |
| Botulinum A toxin a |
| Procainamide a |
| Penicillamine |
| Erythromycin |
| Aminoglycosides |
| Drugs toxic to muscle: |
| Amiodarone a |
| Alcohol a |
| HMG-CoA reductase inhibitors a |
| Cyclosporin |
| Penicillamine |
| Miscellaneous, mechanism presumed neuromyopathic: |
| Digoxin a |
| Trichloroethylene a |
| Vincristine a |
| Drugs inhibiting salivation: a |
| Anticholinergics, antidepressants, antipsychotics, antihistamines, antiparkinsonian drugs, antihypertensives, diuretics |
a Indicates that specific reports of drug-related dysphagia exist.
Myogenic Causes of Oropharyngeal Dysphagia
Myasthenia gravis
Dysphagia affects 30% to 60% of cases of myasthenia. Dysphagia is present at diagnosis in around 20% of cases, and may be the sole presenting symptom in 15% of affected individuals. The so-called “fatigable flaccid dysarthria” manifested by hypernasal speech (velopharyngeal incompetence), imprecise articulation, and breathiness reflects bulbar dysfunction and is usually prominent in those with dysphagia; progressive difficulty chewing and swallowing during the course of a long meal may also be reported. The ocular features of palpebral ptosis and diplopia are usually, but not invariably, present. Diagnosis may be apparent from typical clinical signs including fatigability, but the presentation in the elderly can be atypical, and this treatable condition should be considered in any elderly dysphagic patient even when typical ocular signs are absent.
The diagnosis is confirmed by detecting anti-acetylcholine receptor (AChR) antibodies which are present in 85% of patients. The finding of AChR antibodies is highly specific and virtually diagnostic. In patients who are seronegative for AChR, and in particular those with predominant bulbar or respiratory muscle involvement, anti–muscle-specific tyrosine kinase (anti-MuSK) antibodies can be detected. The edrophonium (Tensilon) stimulation test may be positive but single-fiber electromyographic (EMG) recording is the most sensitive diagnostic test.
The response of pharyngeal swallow dysfunction to an acetylcholinesterase inhibitor and immunosuppressive therapy is variable and may respond less satisfactorily than do other muscle groups. Even in those with a satisfactory clinical response, the videofluoroscopic improvement following therapy may be marginal, which casts doubt on the use of videofluoroscopy in assessing the progress of these patients. Notwithstanding the frequently disappointing response to medical therapy, establishing the diagnosis does influence management as it always warrants drug therapy, a search for thymoma, and avoidance of risk factors for myasthenic crisis, to which the dysphagic patient is frequently exposed, including respiratory tract infections, anesthesia, and surgery.
Inflammatory myopathies
Dysphagia complicates 30% to 60% of cases of inflammatory myopathy (polymyositis, dermatomyositis, and inclusion body myositis). The clinical features of inflammatory myopathy generally include a subacute or chronic and progressive symmetric, proximal, muscular weakness. However, one third of those affected presenting with dysphagia as their only presenting symptom will have no clinically apparent extrabulbar muscular weakness.
Diagnosis maybe confirmed by abnormalities of one or more of muscle enzymes, EMG, or muscle biopsy. Serum creatine phosphokinase (CPK) is the most sensitive enzyme but the CPK level is normal, even in active disease, and in 25% of those with dysphagia as their presenting complaint. Electromyography is useful in excluding neurogenic disorders and will demonstrate features consistent with inflammatory myositis in 85% to 90%. Muscle biopsy is required for definitive diagnosis and to distinguish between dermatomyositis, polymyositis, and inclusion body myositis. Notwithstanding, muscle biopsy is diagnostic in only 80%, emphasizing that diagnosis of myositis can be elusive and the need for complete clinical, biochemical, and laboratory evaluation in suspected cases. The inflammatory process may be patchy and is occasionally confined to the pharyngeal musculature.
The videofluoroscopic features are variable. Pharyngeal dysfunction is almost invariably present and radiographic evidence of aspiration is seen in 60%. Restrictive cricopharyngeal disorders (cricopharyngeal bar, cricopharyngeal stenosis, and Zenker) are more commonly seen in inflammatory myopathy presenting with dysphagia than they are in neurogenic dysphagia. Combined videoradiographic and manometric evaluation of this population showed normal sphincter relaxation, restricted sphincter opening, and raised hypopharyngeal intrabolus pressures ( Fig. 3 ).
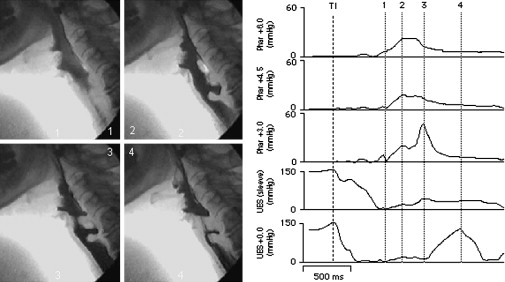
The mainstay of treatment of inflammatory myopathies is immunosuppressive therapy with steroids in the first instance, with azathioprine or methotrexate as second line or steroid-sparing agents. Despite the lack of controlled efficacy trials, a significant number of patients respond favorably to these agents. High-dose intravenous immunoglobulin has been shown to be of clear benefit in a controlled trial for those with polymyositis and dermatomyositis, although inclusion body myositis is generally resistant to standard therapies. Given the common finding of restricted UES opening in this population, cricopharyngeal disruption can be beneficial in at least 50% of patients.
Toxic and metabolic myopathies
Muscle weakness affects 80% of thyrotoxic patients and men are affected more commonly than women. Associated pharyngeal dysphagia is generally slowly progressive and may be the presenting feature of this endocrinopathy. Although uncommon, this condition should always be considered, particularly in the elderly when the more classic thyrotoxic features may be absent, because it is a reversible cause of dysphagia. The pharyngeal dysphagia usually responds well to treatment with restoration of the euthyroid state. Several drugs capable of causing toxic or inflammatory myopathy should be considered in the assessment of the patient with dysphagia, as removal of the drug generally reverses the dysphagia ( Table 2 ).
Presenting Features and Clinical Assessment of Oropharyngeal Dysphagia
Oropharyngeal dysphagia can manifest with one or more symptoms that are specific for oropharyngeal dysfunction and which help the clinician distinguish it from esophageal dysphagia. Swallow initiation may be delayed or absent. Aspiration may manifest as deglutitive cough. Nasopharyngeal regurgitation may be reported. Excessive postswallow residue commonly necessitates repeated swallows to effect pharyngeal clearance. The patient may describe the bolus holding up in the neck but this can be a false localizing feature of esophageal dysphagia and is certainly not specific for pharyngeal dysfunction. It is usual for several of these symptoms to manifest simultaneously in the dysphagic patient. In addition, in the aged population, weight loss, nutritional deficiency, and pneumonia are not uncommon presenting problems.
The circumstances of symptom onset, duration, and progression of dysphagia provide useful diagnostic information. A sudden onset of dysphagia, often in association with other neurologic symptoms or signs, usually indicates a cerebrovascular event. A more insidious onset is more consistent with disorders such as inflammatory myopathy, myasthenia, or amyotrophic lateral sclerosis. Additional neurologic symptoms, when present, such as vertigo, nausea, vomiting, hiccup, hoarseness, tinnitus, diplopia, and so forth, may help localize a lesion to the brainstem. More widespread neuromuscular symptoms such as dysarthria, diplopia, limb weakness, or fatigability might suggest a motor neuronal or myopathic etiology.
The elderly patient with oropharyngeal dysphagia needs a careful neurologic assessment. The diagnostic workup of these patients aims to: (1) identify features of underlying systemic or metabolic disease when present; (2) localize, if possible, the neuroanatomical level and severity of a causative neurologic lesion when present; and (3) detect adverse sequelae such as pulmonary sepsis or nutritional deficiency, which are important indicators of the severity of dysphagia. In addition, an assessment by a speech-language pathologist will provide further information about language, cognitive and behavioral dysfunction, as well as the strength and range of movement of the muscles involved in speech and swallowing. This information will directly influence decisions as to the patient’s suitability for swallow therapy and the type of therapy adopted. An important role of the speech pathologist is to conduct a videofluoroscopic swallow study, usually called a modified barium swallow, to assess aspiration risk and to guide therapy.
Techniques to Evaluate the Patient with Oropharyngeal Dysphagia
Videofluoroscopy
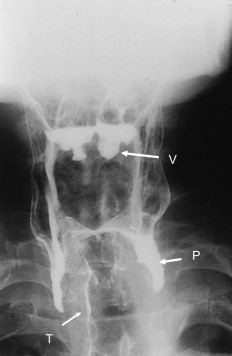
Static films, obtained during a standard barium swallow, may provide important clues to the presence of pharyngeal neuromuscular dysfunction, such as impaired pharyngeal clearance and tracheal aspiration ( Fig. 4 ). A standard barium swallow can readily identify structural causes of dysphagia such as diverticula, webs, stenoses, or cancers. However, static films are inadequate to define the mechanics of the abnormal swallow, which is achieved by performing a modified barium swallow. This test acquires dedicated lateral and anteroposterior views of the oral and pharyngeal phases of the swallow, permits standard and slow motion replay of the swallow to define the mechanisms and severity of dysfunction as well as the influence of modifications to bolus consistency, postures and other swallow maneuvers on bolus flow and clearance. Videofluoroscopy is a sensitive means of confirming oral-pharyngeal dysfunction if its presence is uncertain on the basis of history. This technique provides information on the presence and severity of the major categories of dysfunction, including the presence, timing, and severity of aspiration. Identification of these mechanisms assists the therapist in deciding on specific swallow therapies and the need for nonoral feeding when aspiration is demonstrated.
Nasoendoscopy
Fiberoptic nasoendoscopy is the optimal method for identifying mucosal abnormalities and choosing the site for biopsy, and is mandatory in all cases in which malignancy is suspected. Nasoendoscopy, frequently referred to as fiberoptic endoscopic examination of swallowing (FEES), is less well suited to the assessment of swallow mechanics than videofluoroscopy but can detect the absence of, or profound delay in, initiating the pharyngeal swallow response, and can provide indirect evidence of aspiration.
Manometry
Intraluminal manometry can quantify pharyngeal deglutitive forces, detect failure of UES relaxation, and the relative coordination of pharyngeal contraction with UES relaxation. The age-related changes in manometric parameters dictate the need for comparison with appropriate normative values. It is often useful to combine manometry with videofluoroscopy. This technique permits correlation of the motion of anatomic structures with the resulting intraluminal pressures and the identification of intrabolus pressure, which is an indirect measure of UES compliance. Failure of UES relaxation, which can only be reliably determined by manometry or EMG, indicates a rostral medullary lesion or Parkinson disease. Identification of certain manometric abnormalities, particularly failed UES relaxation or elevated intrabolus pressure, may help in diagnosis and may influence management decisions, particularly relating to the advisability of cricopharyngeal myotomy or dilatation. However, it remains to be proven that such intervention in this context influences clinical outcome.
Management of Oropharyngeal Dysphagia
Management principles
Broadly speaking, the aims of management are to identify and treat an underlying primary disease if possible, and then try to compensate for or circumvent the specific mechanical disturbances responsible for the dysphagia and to eliminate or minimize aspiration if present.
First, confirm that oropharyngeal dysphagia is indeed a problem and attempt to identify the underlying cause. A careful history will generally distinguish oropharyngeal dysphagia from globus, xerostomia, and esophageal dysphagia. History and physical examination may provide clues of a treatable systemic, metabolic, or drug-related disorder. This examination should include a “dysphagia screen” of laboratory tests to exclude systemic, metabolic, or directly treatable neuromyogenic diseases (eg, thyrotoxicosis, myasthenia, myositis). This laboratory screen includes CPK, erythrocyte sedimentation rate (ESR), thyroid function tests, and AChR antibodies (and, if negative, MuSK antibodies; see earlier). A cerebral MRI or CT scan is frequently indicated if a cerebrovascular event is suspected.
Second, identify the structural or neuromyogenic mechanisms of oropharyngeal dysfunction. Structural disorders are generally readily detected by radiographic or endoscopic evaluation. Identification of a neoplasm or a Zenker diverticulum will dictate surgery. A cervical web or a cricopharyngeal stenosis will prompt dilatation or, in some cases, cricopharyngeal myotomy. Nasoendoscopic examination of the laryngopharynx is mandatory if neoplasm is suspected. In the context of disorders with a high prevalence of failed UES relaxation, such as Parkinson disease and medullary lesions, pharyngeal manometry, with or without concurrent videofluoroscopy, may detect failed UES relaxation, which might prompt consideration for cricopharyngeal myotomy.
Third, determine the risk of aspiration pneumonia, which is the primary factor in the decision as to whether and when nonoral feeding should be instituted. The risk of aspiration is best determined by the modified barium swallow examination, as this risk is underestimated by about 50% by clinical assessment alone. The decision on the advisability of gastrostomy feeding is also influenced by the likelihood that therapeutic maneuvers, which may be tested during videofluoroscopy, will reduce or eliminate aspiration; the natural history of the underlying disease; and the patient’s cognitive ability.
Finally, after exclusion of structural lesions and underlying treatable diseases, and having established the safety of oral feeding, specific “local” therapy should be considered. The therapeutic options open are dietary modification, swallow therapy, or surgery, or a combination of all three.
Dietary modification and swallow therapy
Therapeutic strategies include dietary modification, manipulation of swallowing posture, or swallowing technique. Modifications of swallowing technique are intended to strengthen weak oropharyngeal muscle groups, thereby improving their speed and range of movement, or to selectively modify the mechanics of the swallow to facilitate bolus flow and minimize aspiration. In applying swallow therapies the speech-language pathologist will use videofluoroscopy to define the relevant mechanism of dysfunction and examine the acute effects of therapeutic strategies designed to eliminate or compensate for that dysfunction. Simple dietary modification has been shown in a single, randomized, controlled trial to reduce the risk of aspiration pneumonia, and should be instituted if an aspiration risk is apparent. Most of the studies in this field have focused on poststroke dysphagia. There are reasonable data supporting the biologic plausibility of the remaining swallow strategies, but the limited available controlled efficacy data are inconclusive. There is only one published randomized controlled trial of swallow behavioral therapy. Twelve months after acute stroke, 15% of that study population developed one or more of the study end points, thus failing to show benefit for intensive swallow rehabilitation. On the other hand, swallow therapy has not been proven to be ineffective, and, based on the demonstration of biologic plausibility for specific therapeutic techniques, the consistency of the low grade evidence suggesting efficacy, the low cost, and the absence of either risk or any better alternative in many instances, it is appropriate to institute swallow therapy under the supervision of a speech-language pathologist. Large-scale controlled trials are necessary to clarify the appropriateness of all current treatment strategies in neurogenic oropharyngeal dysphagia.
Enteral tube feeding
PEG tubes, or nasogastric tubes (NGTs), are frequently used to feed stroke patients enterally to prevent or minimize the risk of aspiration pneumonia. There are five studies comparing the two techniques. Whereas PEG seems to be superior in terms of reliable delivery of prescribed calories, PEG was not found to be superior to NGT in preventing aspiration, in reducing mortality, nor poor outcome at 6 months in dysphagic patients following stroke. Of some concern, the largest prospective randomized controlled trial, comparing PEG with NGT feeding, found PEG feeding to be associated with a 7% higher risk of death or poor outcome at 6 months. On the basis of available evidence, a reasonable approach in patients with stroke-related dysphagia who have aspiration demonstrated radiographically on all bolus consistencies trialed, is to place an NGT in the first instance. Because of the substantial spontaneous recovery rate following stroke, if the NGT is well tolerated, and if the patient does not habitually remove the tube, then the swallow function and aspiration risk can be re-evaluated by repeat modified barium swallow in 3 to 4 weeks. If the aspiration risk persists, the NGT can be replaced by a PEG tube. This recommendation may need to be modified on a case-by-case basis and under specific circumstances. For example, hospital and nursing policies vary greatly among specific rehabilitation and aged care facilities. Unfortunately, in some cases these policies are not evidence-based but are driven by health economics.
Surgical options
The indications for surgery for structural abnormalities are discussed above. Surgical approaches may be considered in neuromyogenic dysphagia if time-related recovery or treatment of specific neuromyogenic disease has been disappointing. Several indicators have been proposed that might predict a favorable outcome from cricopharyngeal myotomy, such as: intact swallow initiation, “adequate” lingual and pharyngeal propulsive forces, radiographic or manometric evidence of increased outflow resistance at the UES (failed relaxation or constriction), and a good prognosis for the underlying neurologic disease. Although the data supporting the efficacy of cricopharyngeal myotomy for structural cricopharyngeal disorders are strong, the outcome following myotomy for neuromyogenic dysphagia is far less certain. There are no controlled trials of cricopharyngeal myotomy in neurogenic dysphagia, but the available evidence suggests an overall response rate of around 60% with an operative mortality of 1% to 2%.
Most surgical procedures in the dysphagic patient aim to reduce or eliminate aspiration. The more conservative procedures (laryngeal suspension, vocal fold augmentation or medialization, and epiglottoplasty) preserve voice. The more destructive procedures, which achieve tracheo-esophageal separation, render the patient unable to phonate. Such procedures include glottic closure, tracheo-esophageal diversion, laryngotracheal separation, and total laryngectomy. There is virtually no reliable efficacy data on which to base recommendations and indications for such therapies.
Botulinum toxin injection
Botox injection, either endoscopically or transcutaneously, has been reported in small case series to be of benefit. Although these studies attempted to target cricopharyngeal disorders, optimal identification of failed cricopharyngeal relaxation was not adopted in those studies. Diffusion of the toxin to adjacent muscles may worsen dysphagia or cause vocal-cord dysfunction. Controlled trials of the safety and efficacy of Botox is required before it might be used to target particular dysphagic populations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







