The primary goal of most colonoscopies, whether performed for screening, surveillance, or diagnostic examinations (those performed for symptoms or positive screening tests other than colonoscopy) is the detection of neoplasia and its subsequent removal by either endoscopic polypectomy or referral for surgical resection. Unfortunately, colonoscopy has proved to be a highly operator-dependent procedure with regard to detection. Variable detection results in some of the cancers that occur in the interval before the next colonoscopy.
Key points
- •
Advanced colonoscopy skill must be demonstrated by measurement of quality indications, particularly the adenoma detection rate.
- •
High-level detection is associated with white-light examination technique that involves adequate time; meticulous probing of the proximal sides of folds, flexures, and valves; adequate colonic distention; and effort to clean up residual debris.
- •
Endoscopists should be able to recognize the full spectrum of pre-cancerous colorectal lesions, including subtle lesions such as flat and depressed conventional adenomas and serrated lesions.
- •
New approaches to reducing variable detection, including the use of systematic video recording of examinations, correction of visual gaze patterns, and development of insights into personality and behavioral factors that influence detection, warrant aggressive investigation.
Introduction
The primary goal of most colonoscopies, whether performed for screening, surveillance, or diagnostic examinations (those performed for symptoms or positive screening tests other than colonoscopy) is the detection of neoplasia and its subsequent removal by either endoscopic polypectomy or referral for surgical resection. Unfortunately, colonoscopy has proved to be a highly operator-dependent procedure with regard to detection, and the consequences of this variation include occurrence of colorectal cancer in the interval before the next colonoscopy (so-called “interval cancers” or “post-colonoscopy cancers”). Many of the articles in this edition of Gastroenterology Clinics of North America are devoted to technical improvements in imaging that could potentially improve neoplasia detection or reduce the variation between examiners and detection. However, studies describing attempts to improve detection solely through technology improvements have generally shown lower detection gains, than is achieved by taking the colonoscope out of the hands of a low-level adenoma detector and giving it to a high-level detector. A high-level detector will commonly increase the percentage of patients with one or more adenomas detected by 3-fold to 6-fold and the total number of adenomas detected by more than 10-fold. The goal of this article is to help create colonoscopists who can achieve high-level detection of the full spectrum of cancerous and precancerous lesions in the colorectum.
Introduction
The primary goal of most colonoscopies, whether performed for screening, surveillance, or diagnostic examinations (those performed for symptoms or positive screening tests other than colonoscopy) is the detection of neoplasia and its subsequent removal by either endoscopic polypectomy or referral for surgical resection. Unfortunately, colonoscopy has proved to be a highly operator-dependent procedure with regard to detection, and the consequences of this variation include occurrence of colorectal cancer in the interval before the next colonoscopy (so-called “interval cancers” or “post-colonoscopy cancers”). Many of the articles in this edition of Gastroenterology Clinics of North America are devoted to technical improvements in imaging that could potentially improve neoplasia detection or reduce the variation between examiners and detection. However, studies describing attempts to improve detection solely through technology improvements have generally shown lower detection gains, than is achieved by taking the colonoscope out of the hands of a low-level adenoma detector and giving it to a high-level detector. A high-level detector will commonly increase the percentage of patients with one or more adenomas detected by 3-fold to 6-fold and the total number of adenomas detected by more than 10-fold. The goal of this article is to help create colonoscopists who can achieve high-level detection of the full spectrum of cancerous and precancerous lesions in the colorectum.
Paris classification
Both malignant and precancerous lesions in the colon grow in a spectrum of shapes that range from overtly polypoid or fungating to lesions that have no elevated component and are entirely or mostly depressed below the level of normal mucosa. Without doubt, lesions that are flat or depressed are more difficult on average to detect and are more easily hidden from view behind folds and flexures, as well as overlooked even when brought into view, compared to the polypoid lesions. In order to study and communicate effectively regarding the spectrum of shapes of cancer and precancerous lesions, the Paris Classification was developed.
In the Paris Classification ( Fig. 1 ), lesions are divided into polyps (Type 1 lesions) and flat and depressed lesions (Type 2). Type 1 lesions may be pedunculated (1p) or sessile (1s) polyps. Among the polyps, the sessile polyps are substantially more common than pedunculated polyps.
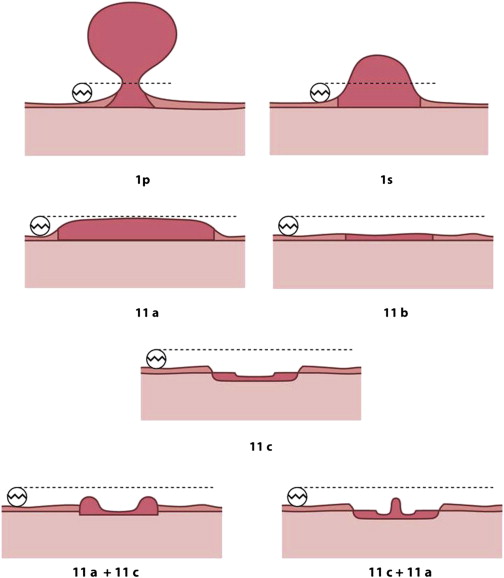
Among the Type 2 lesions, the most common by far is the 2a lesion ( Fig. 2 ), which is flat but elevated relative to the mucosal surface. The difference between a 1s lesion and a 2a lesion is that a 1s lesion projects into the lumen more than the diameter of a standard biopsy forceps of 2.5 mm and the flat 2a lesions project into the lumen less than 2.5 mm. 2b lesions are truly flat and are exceedingly rare. In my experience, most 2b lesions are serrated rather than conventional adenomas. Both the polypoid lesions (1p and 1s) and the flat lesions (2a and 2b) have a low prevalence of invasive cancer. Although there is a widely held impression that flat lesions are more likely to contain high-grade dysplasia and invasive cancer than polypoid lesions, literature on that issue is quite mixed, and some studies show that 2a lesions have the lowest prevalence of invasive cancer of all precancerous lesions.
Depressed lesions, designated as 2c and its variants in the Paris Classification (see Fig. 1 ) are some of the most dangerous lesions in the colorectum. These lesions are characterized by a depressed element that typically occupies most or a substantial part of the lesion surface and has a fairly sharp drop-off from the normal mucosa or elevated portion of the lesion to the depressed section. The shape of an individual lesion and its proper assignment in the Paris Classification can often be best achieved by surface dye spraying with dilute indigo carmine, which highlights lesion morphology. The prevalence of depressed lesions is quite low, approaching rarity, but the lesions are quite significant because in many series up to 50% contain high-grade dysplasia or invasive cancer. Correct recognition of depressed lesions is also important because if possible they should be resected en bloc and with inclusion of a rim of normal mucosa at the periphery.
The most common area of confusion is distinguishing a 2a plus 2c depressed lesion ( Fig. 3 ) from a 2a lesion that has a slight valley on its surface ( Fig. 4 ), commonly referred to as a “pseudodepression” or “2a dip.” About 15% of 2a adenomas have such a dip and it does not predict an increased chance of advanced pathology. The characteristic features are that the 2a dip occupies only a small portion of the surface area of the 2a adenoma, and the transition from the elevated portion into the valley is gradual in the 2a dip, as opposed to sharp in the 2a plus 2c depressed lesion.
When 2a lesions extend over 1.0 cm in diameter, they are referred to as lateral-spreading tumors (LSTs). In the past, such lesions were often referred to as “carpet lesions” or just “sessile polyps.” LSTs are further characterized as granular LST, meaning that the surface is bumpy, or nongranular LST, which has a smooth surface. Granular LSTs are considerably more common than nongranular and also are much less likely to contain high-grade dysplasia or invasive cancer compared with nongranular LSTs.
Spectrum of disease
The modern colonoscopist should understand the molecular basis of colorectal cancer as it relates to the spectrum of precancerous lesions that must be detected and removed during colonoscopy. It is often said that the molecular profiles of any 2 colorectal cancers are never identical. However, 3 broad categories of molecular pathways have been described ( Table 1 ) and have clinical implications for the colonoscopist.
| Pathway | Frequency | Genes | MSI | Precursor | Speed |
|---|---|---|---|---|---|
| CIN | 65%–70% | APC K-ras p53 | No | Adenoma | Slow |
| Lynch | 3% | MLH1 MLH2 MLH6 PMS2 | Yes | Adenoma | Fast |
| CIMP | 30%–35% | BRAF | Sometimes | Serrated | Can be fast |
The most common molecular pathway is the chromosomal instability (CIN) pathway, in which cancers arise through the conventional adenomatous polyp. Adenomas carry point mutations in tumor suppressor genes and oncogenes, which commonly include the adenomatous polyposis coli gene, the k-ras oncogene, and the p53 tumor suppressor gene (see Table 1 ). Conventional adenomas are classified as tubular, tubulovillous, or villous, and the tubulovillous and villous adenomas are said to contain “villous elements.” Adenomas with villous elements have a greater chance of having high-grade dysplasia or invasive cancer compared with tubular adenomas. All conventional adenomas by definition are dysplastic, which should be classified as low grade or high grade. In the CIN pathway, the passage of an adenoma from low-grade to high-grade dysplasia and then to invasive cancer is believed to typically require 10 to 20 years, although the process may occur more quickly in elderly patients whose adenomas may have accumulated more mutations. However, because the dwell time of small tubular adenomas is typically decades, the consequences of missing a small (6–9 mm) or diminutive (1–5 mm) tubular conventional adenoma are almost always minimal.
The least common of the molecular pathways is the Lynch syndrome, in which patients are born with inherited germline mutations in 1 of the 4 mismatch repair genes ( MLH1, MSH2, MSH6, PMS2 ). Patients carrying these mutations are prone to deletions in short repeating sequences of DNA called microsatellites. When these errors are not repaired, the result is referred to as microsatellite instability. Tumor suppressor genes and oncogenes with microsatellites in their coding regions are susceptible to mutation in patients with Lynch syndrome. Mutations normally occur at a relatively high rate but are repaired by the mismatch repair system; in Lynch syndrome, there is failure to repair these mutations, and patients can accumulate mutations in cancer genes faster than this process occurs in normal individuals. The result is the potential for even a small adenoma to transform into cancer in a few years. Therefore, the goal of detection in patients with Lynch syndrome is the complete clearing of all precancerous lesions, including tiny conventional adenomas.
The second most important molecular pathway from a quantitative perspective is also the most recent to be recognized. This pathway is often designated the “serrated pathway” or the “hypermethylation pathway” or “CIMP-high” pathway, referring to the CpG island methylator phenotype (CIMP). These tumors account for about 30% of all colorectal cancers and are distributed toward the proximal colon. Rather than mutation in the k-ras oncogene, they typically carry mutations in the BRAF oncogene. BRAF is in the same signaling pathway as k-ras, and concomitant mutations in both k-ras and BRAF are distinctly uncommon in colorectal cancers, occurring in only 1% of tumors. BRAF mutation is almost synonymous with CIMP-high colorectal cancer. Approximately half of CIMP-high colorectal cancers are microsatellite unstable, and CIMP-high tumors account for about 80% of all microsatellite unstable colorectal cancers. The origin of microsatellite instability in CIMP-high tumors is epigenetic inactivation of the MLH1 gene (1 of the 4 mismatch repair genes) by hypermethylation of its promoter region.
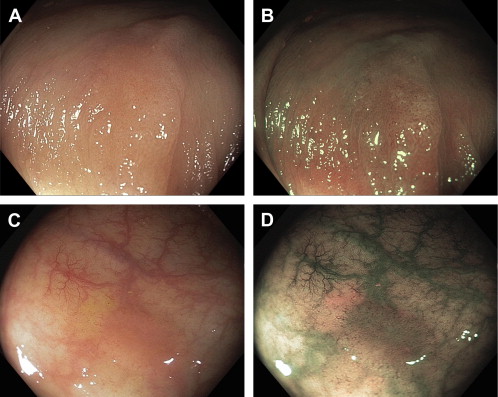
From the colonoscopist’s perspective, the precursor of the hypermethylated tumors is not the conventional adenoma but rather a group of lesions called “serrated.” The World Health Organization advises that serrated lesions be grouped into 3 major histologic categories ( Box 1 ), which includes hyperplastic polyps, sessile serrated polyps (synonymous with sessile serrated adenomas), and traditional serrated adenomas. The principle precursor of hypermethylated tumors is the sessile serrated polyp, which shares with hypermethylated tumors proximal colon location, hypermethylation, and BRAF mutation (see Box 1 ). Serrated lesions can be readily distinguished from conventional adenomas during endoscopy. They have a color similar to the surrounding mucosa, indistinct edges, no or few blood vessels on the surface, and their presence is often signaled by either a “mucus cap” or collection of debris, which often accumulates near the lesion edge ( Fig. 5 ).
Hyperplastic polyp
Sessile serrated polyp (same as sessile serrated adenoma)
Without cytologic dysplasia
With cytologic dysplasia
Traditional serrated adenoma







