Fig. 11.1
Computed tomography scan of patient with BRPC due to hepatic arterial involvement both before (a) and after (b) neoadjuvant therapy with FOLFIRINOX. The tumor can be seen encasing the gastroduodenal artery (GDA) in both images. It extended up the GDA and directly contacted the hepatic artery, and this did not change following neoadjuvant treatment
Anatomic Factors
Neoadjuvant therapy should be offered to all patients with anatomic BRPC as defined in “Introduction.” These patients are highly likely to require major vascular resection and reconstruction and by definition are at increased risk for a margin-positive resection. This is particularly true for patients with arterial involvement. In such cases, even focal or localized involvement can be associated with microscopic extension of cancer cells along the periarterial lymphatic and neural plexuses that ultimately result in a microscopically positive resection margin. This is not typically true for patients with BRPC due to isolated vein involvement. While we prefer neoadjuvant therapy for all patients with BRPC, a surgery-first approach may be a reasonable option for fit patients with isolated vein involvement, especially those who are jaundiced and would require endoscopic or percutaneous biliary drainage in order to receive neoadjuvant treatment, and those in whom a tissue diagnosis cannot be obtained.
Disease Biology
In addition to patient and tumor- related factors, disease biology must be considered in decisions regarding timing of surgery. Patients with clinical evidence of regional lymph node metastasis and those with markedly elevated serum CA 19-9 levels at presentation are more likely to harbor radiographically occult distant metastasis. These patients are at risk of developing early systemic recurrence following surgery and should be treated with systemic therapy first.
Summary
Once patient, tumor, and disease biology-related factors have been assessed, an experienced surgeon can make a recommendation regarding the optimal timing of surgery for a patient with BRPC. This recommendation should be discussed in a multidisciplinary setting. In cases where neoadjuvant therapy is recommended, available regimens, including clinical trials, should be discussed and the timing of re-staging and surgical follow-up should be established.
Operative Principles
Whether patients receive neoadjuvant therapy or upfront surgical resection, the principles of surgery for BRPC are the same. The goal of surgery is complete, R0 resection with minimal morbidity. Neoadjuvant therapy is thought to improve the likelihood of R0 resection for BRPC, but it rarely changes the extent of resection necessary [1]. As illustrated in Fig. 11.2, if patients have evidence of vascular involvement at presentation, the need for vascular resection and reconstruction is unlikely to change the following neoadjuvant treatment. This chapter discusses basic operative principles of pancreatic resection and summarizes current data regarding variations in surgical technique and perioperative care that have been proposed to minimize morbidity. Techniques of vascular resection and reconstruction will be discussed in subsequent chapters.
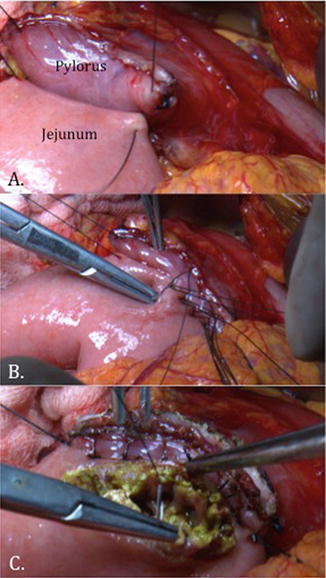

Fig. 11.2
Intraoperative photograph of reconstruction during pylorus-preserving pancreaticoduodenectomy. (a) The duodenum is divided just distal to the pylorus and is approximated to the jejunal limb in an end-to-side fashion. (b, c) A hand-sewn, double-layered duodenojejunostomy is performed
Staging Laparoscopy
Laparoscopy has been investigated as a staging tool in pancreatic cancer given the high incidence of synchronous metastasis and the poor sensitivity of cross-sectional imaging for detection of small-volume peritoneal surface lesions. Staging laparoscopy (SL) consists of gross inspection of the peritoneal cavity with biopsy of any abnormal or suspicious appearing lesions and can be performed under the same anesthetic as surgical resection, or can be performed as a separate procedure prior to planned resection. If performed separately, peritoneal washings can be obtained for cytologic analysis. Positive peritoneal cytology is considered M1 disease.
Multiple studies have been conducted evaluating the yield of SL for the detection of radiographically occult metastatic disease in patients with pancreatic cancer. The overall yield of detection of radiographically occult metastasis ranges from 14 to 30 % [2–5]. Most of these studies consisted of heterogeneous patient populations, however, including patients with both resectable and borderline resectable disease, and some included patients with histological diagnoses other than pancreatic cancer. Improvements in imaging are ongoing and thus the true yield of SL and cost-effectiveness is difficult to determine accurately [5]. As a result, the routine use of SL for patients with pancreatic cancer has not been widely adopted.
The yield of SL in patients with BRPC is likely higher. Most practitioners agree that there is a role for the selective use of SL in patients with pancreatic cancer at high-risk for occult metastasis and who do not otherwise require a laparotomy for any type of palliative procedure. This includes patients with BRPC, body/tail tumors which tend to present at a later stage, tumors >3 cm in diameter, elevated CA 19-9 (>4 times upper limit of normal), enlarged regional lymph nodes, and findings concerning but equivocal for metastases on cross-sectional imaging [6, 7]. Additionally, SL with peritoneal washings should be strongly considered for patients with BRPC for whom neoadjuvant therapy is planned, particularly if on a clinical trial. SL may also be useful to rule out radiographically occult systemic disease prior to the administration of radiotherapy, if that modality is being used.
Pancreatic Resection
Surgical options for pancreatic adenocarcinoma include distal pancreatectomy with splenectomy (DP), pancreaticoduodenectomy (PD), and in rare cases, total pancreatectomy (TP). Lesser resections such as enucleation, central pancreatectomy, and spleen-preserving distal pancreatectomy do not achieve a sufficient regional lymphadenectomy and are not recommended for pancreatic adenocarcinoma.
Distal Pancreatectomy with Splenectomy
DP with en bloc splenectomy is indicated for tumors of the body and tail of the pancreas. These tumors are generally more advanced at presentation than tumors arising in the head of the gland, because they do not result in jaundice and therefore usually present with pain and/or weight loss when they are large. The first step in DP is entering the lesser sac by opening the gastrocolic ligament. This dissection is extended up along the greater curvature of the stomach through the short gastric vessels, to the left crus of the diaphragm. There are usually filmy attachments between the posterior wall of the stomach and the capsule of the pancreas that need to be divided.
Once the body and tail of the pancreas are exposed, the lesion is identified. This may be obvious in cases of large masses, but may require intraoperative ultrasound for small, incidentally identified lesions. A pancreatic transection site proximal to the lesion, ideally several centimeters away, is then selected. The inferior border of the pancreas, which is generally an avascular plane, is then mobilized. The splenic artery is identified at its origin from the celiac axis and should be encircled with a vessel loop. The splenic artery and vein may be divided en bloc with the pancreas for very distal tumors, but often need to be divided at their origins, outside of the pancreas for more proximally located tumors. In this case, the artery should be divided first. For large, bulky tumors, it may be helpful to mobilize the lateral attachments of the spleen as the first step so that the spleen and distal pancreas can be rotated up and extracorporealized to facilitate the posterior dissection. For tumors invading the stomach, left adrenal gland, or transverse mesocolon, en bloc resections of these organs should be performed to achieve clear margins. The inferior mesenteric vein may or may not need to be ligated and divided, depending on its insertion site along the superior mesenteric vein (SMV)/splenic vein.
The technique of radical antegrade modular pancreatosplenectomy (RAMPS) was first proposed in 2003 as a means of ensuring microscopically negative tangential margins and adequate lymphadenectomy [8]. RAMPS is a modification of standard DP/splenectomy that entails early division of the neck of the pancreas and splenic vessels, lymphadenectomy including periportal, hepatic artery, and celiac axis nodes, and posterior plane of dissection along the anterior surface of the adrenal gland and including Gerota’s fascia. Given the lack of controlled data and the predominant systemic recurrence pattern of pancreatic cancer, the relative value of this approach remains unclear.
A topic of debate regarding DP is focused on the best method of transection of the pancreas to minimize the rate of leaking of pancreatic exocrine fluid from the divided stump. Leaks lead to intra-abdominal fluid collections, abscesses, and once drained, pancreatic fistula (PF). Multiple techniques have been investigated, including sharp or cautery transection with oversewing of the stump, use of fibrin glue, and soft tissue reinforcement with omental or falciform patches, and stapled transection with or without bioabsorbable mesh reinforcement. None of these methods have been definitively shown to be superior to others.
Staple line reinforcement with bioabsorbable mesh has been investigated in multiple retrospective studies with conflicting results [9–11]. These studies prompted a randomized, prospective trial comparing stapled transection of the pancreas with or without bioabsorbable mesh, which demonstrated a significant decrease in clinically relevant PF (CR-PF) with use of mesh reinforcement (2 % vs. 20 %, p < 0.001) [12]. Despite these findings, this technique has not been widely accepted. Information on pancreatic duct diameter and parenchymal consistency in the two groups was not reported, and many question the validity of the results given the extremely low rate of PF in the mesh group. Decisions regarding the optimal method of transection should be made at the time of surgery. We prefer stapled transection with bioabsorbable reinforcement for soft, normal-textured glands, and oversewing for thick, firm glands that cannot be compressed with a stapler.
Pancreaticoduodenectomy
PD is the most commonly performed operation for pancreatic adenocarcinoma, as 60–70 % of tumors arise in the head of the pancreas, and is the primary focus of this chapter. PD is a technically challenging and complex procedure. Since it was first performed as a two-stage operation by Dr. Allen O. Whipple in 1935, significant advances have been made in surgical technique and perioperative care. Operative mortality is now generally <3 % at high-volume centers, but postoperative morbidity remains high, on the order of 30–50 % [13, 14]. In addition to general infectious and cardiopulmonary complications that can occur after any major surgery, well-defined complications associated particularly with PD include delayed gastric emptying (DGE), PF, and post-pancreatectomy hemorrhage (PPH) [15–17]. These definitions are summarized in Table 11.1.
Table 11.1
International Study Group of Pancreatic Surgery definitions of characteristic post-pancreatectomy complications
Complication | Definition | Grade A | Grade B | Grade C |
|---|---|---|---|---|
DGE [15] | Inability to return to a standard diet by the end of the first postoperative week, in the absence of mechanical obstruction | NGT still required 4–7 days after surgery, or NGT reinserted on or after postoperative day #3 | NGT still required 8–14 days after surgery, or NGT reinserted on or after postoperative day #7 | NGT still required >14 days after surgery, or NGT reinserted on or after postoperative day #14 |
PF [14] | Drain output of any measurable volume on or after postoperative day #3 with amylase content >3 times the serum amylase | |||
Clinical condition | Well | Often well | Appearing ill | |
Specific treatment required | No | Yes/no | Yes | |
US/CT (if obtained) | Negative | Negative/positive | Positive | |
Persistent drainage (>3 weeks) | No | Usually yes | Yes | |
Reoperation | No | No | Yes | |
Death related to PF | No | No | Possibly yes | |
Infection | No | Yes | Yes | |
Sepsis | No | No | Yes | |
Readmission | No | Yes/no | Yes/no | |
PPH [16] | Intra- or extraluminal hemorrhage following pancreatic resection | |||
Timing and severity | Early (≤24 h) and mild | Early and severe OR late (>24 h) and mild | Late and severe | |
Clinical condition | Well | Often well | Severely impaired, life-threatening | |
Clinical consequence | Observation, CBC, US, ±CT | Observation, CBC, CT, angiography; requiring transfusion, ICU care, diagnostic or therapeutic endoscopy, embolization, or re-laparotomy for early bleeding | Angiography, CT, ±endoscopy or re-laparotomy, ICU care | |
Standard PD involves removal of the head of the pancreas, antrum of the stomach, duodenum, gallbladder, common bile duct, and regional lymph nodes. Regional nodes include those of the porta hepatis to the right of the hepatoduodenal ligament, those in the retroperitoneum to the right of the superior mesenteric artery (SMA), and the anterior and posterior pancreaticoduodenal nodes. The hepatic artery lymph node is also frequently included in the resection as removal of this node facilitates exposure of the gastroduodenal artery (GDA). There is no consensus on a minimum number of nodes to be considered adequate for staging, but different groups have recommended numbers ranging from 11 to 17. Extended lymphadenectomy including aortocaval nodes has been investigated in prospective, randomized trials but has not been associated with a survival benefit. Extended lymphadenectomy has, however, been associated with increased morbidity [7, 18, 19]. Extended lymphadenectomy is therefore not recommended.
Consideration of the precise location of the tumor in the pancreatic head should be made to determine the area at highest risk for a positive margin. For tumors in the pancreatic head near the ampulla and those encroaching on the pancreatic neck, the common bile duct and pancreatic neck margins are at greatest risk for positivity; respectively, and should be assessed with frozen section intraoperatively. Intraoperative assessment of the pancreatic margin is not without controversy, however, as some studies suggest that re-resection does not change outcome, as margin status may be largely reflective of aggressive tumor biology [20]. Our general practice is to resect a positive pancreatic margin a single time. If persistently positive, it is unlikely that proceeding to more radical resection or total pancreatectomy will alter oncologic outcomes. For uncinate process tumors, the retroperitoneal margin is at greatest risk; hence the SMA should be skeletonized to its adventitia. The superior mesenteric and portal veins should be completely mobilized off of the uncinate process first. The medial dissection is then extended to the adventitia of the SMA to maximize the retroperitoneal margin. If there is invasion of the SMV/PV, the SMA, or the hepatic artery by tumor, the area of vascular involvement should be left as the last point of attachment of the specimen so that proximal and distal control of the involved vessel can be achieved prior to reconstruction.
The most common reconstruction technique includes an end-to-side pancreaticojejunostomy (PJ) to the limb of proximal jejunum brought up in a retrocolic position, an end-to-side hepaticojejunostomy approximately 10 cm downstream from that, and an end-to-side gastrojejunostomy 20–30 cm downstream from that in a retro- or ante-colic position. Beyond these basic principles, multiple variations in technique of pancreatic resection and reconstruction have been investigated with the aim of reducing the incidence of postoperative complications .
Pylorus Preservation
PD with preservation of the pylorus, thereby dividing the proximal duodenum just distal to the pyloric ring, was popularized in the late 1970s. A duodenojejunostomy is then created, rather than a gastrojejunostomy (Fig. 11.2). Proposed benefits of this modification are improved digestive function and postoperative nutritional status, and decreased marginal ulceration. Concerns raised regarding pylorus preservation include increased incidence of DGE and inadequate clearance of peripyloric lymph nodes. Multiple randomized, prospective trials have been conducted comparing standard PD with pylorus-preserving PD (PPPD), the most recent of which are summarized in Table 11.2 [21–23]. In 2004, Tran and colleagues reported a prospective, randomized trial of 170 patients who underwent standard (n = 83) or PPPD (n = 87). The two groups were well matched for age, gender, tumor location, and stage. Two patients who were randomized to PPPD underwent standard PD due to suspicion of duodenal involvement and were included in the PPPD group for intention-to-treat analysis. There were no differences in median blood loss, operative time, DGE, or margin status between the groups. Postoperative weight loss was slightly greater in the PPPD group. On subset analysis of patients with adenocarcinoma, there were no differences in median disease-free or overall survival. The authors concluded that both techniques are equally effective.
Table 11.2
Summary of recent randomized prospective studies evaluating pylorus-preserving vs. standard pancreaticoduodenectomy
Study | N | DGE (%) | P | ≥Grade 3 Morbidity | P | Mortality | P | |
|---|---|---|---|---|---|---|---|---|
Mastumoto et al. [20] | PPPD | 50 | 20 | 0.41 | 16 % | 1.00 | 0 | 1.00 |
SPD | 50 | 12 | 14 % | 0 | ||||
Kawai et al. [19] | PPPD | 64 | 17 | 0.02 | – | NS | 0 | 0.99 |
*SPD | 66 | 5 | – | 1 | ||||
Tran et al. [21] | PPPD | 87 | 23 | 0.80 | – | NS | 3 | 0.27 |
SPD | 83 | 22 | – | 6 |
In 2011, Kawai and colleagues compared PPPD with PD dividing the stomach just proximal to the pylorus, thereby preserving more stomach than what is typically done in classic PD. This was also a randomized, prospective study and included 130 total patients (64 PPPD vs. 66 PD with pylorus resection) who were well matched for baseline characteristics. The authors found a statistically significant increase in DGE in the PPPD group (17.2 % vs. 4.5 %, p = 0.02). There were no differences in other study endpoints including postoperative complications, mortality, quality of life, and nutritional status over a 6-month period following surgery. Oncologic outcomes were not reported. The authors concluded that resection of the pyloric ring may reduce DGE compared to PPPD.
Most recently, in 2014, Matsumoto reported 100 patients randomized to PPPD (n = 50) or standard PD (n = 50). The incidence of DGE was 20 % in the PPPD group compared to 12 % with standard PD, and this was not statistically significant (p = 0.41). The study was powered to detect a 20 % difference in DGE rates. There were also no differences in the secondary endpoints, including postoperative complications, morbidity, long-term nutritional status, and diabetic status. Oncologic outcomes were not reported.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







