19
Oesophageal emergencies
Perforation of the oesophagus
Aetiology and pathophysiology
Iatrogenic perforation of the oesophagus
Iatrogenic damage to the oesophagus leading to full-thickness disruption occurs from within in 60–70% of cases, such as during endoscopic instrumentation, or from without, such as during para-oesophageal surgery. Although flexible video endoscopy is safe and has almost totally replaced rigid oesophagoscopy (0.03% perforation risk compared to 0.11% for rigid endoscopy), the dramatic increase in the number of examinations performed has led to an increase in the number of associated injuries. Intubation of the oesophagus can cause proximal perforation with risk increased by hyper-extension of the neck and the presence of arthritic cervical osteophytes or an oesophageal diverticulum. However, in 75–90% of diagnostic cases, trauma is sustained to the distal oesophagus, often in conjunction with an abnormality (Table 19.1). Therapeutic endoscopy carries a significantly higher perforation risk (200-fold), around 5%, that is further increased in patients who have received prior radiotherapy or chemotherapy (as the majority of therapeutic endoscopy is for palliation). Dilatation accounts for the majority of injuries and with a lower risk of perforation when placing self-expanding metal stents. Benign pneumatic dilatation for achalasia carries a higher risk than graded dilatation, due to higher pressures and large balloon size.1 Transoesophageal echocardiography carries risk not only for perforation during blind placement but also when placed for perioperative monitoring due to pressure necrosis. Similarly, any intubation such as placement of a nasogastric tube or inadvertent oesophageal placement of an endotracheal tube may all cause direct trauma. A case review of 75 patients with iatrogenic perforation of the oesophagus reported a not insubstantial overall mortality rate of 19%. Prevention is therefore the best solution, with increasing awareness and training likely to reduce the incidence.2
Table 19.1
Risk of iatrogenic oesophageal disruption through instrumentation
| Medical instrumentation | Percentage risk of iatrogenic oesophageal disruption |
| Dilatation | 0.5 |
| Dilatation for achalasia | 2 |
| Endoscopic thermal therapy | 1–2 |
| Treatment of variceal bleeding | 1–6 |
| Endoscopic laser therapy | 1–5 |
| Photodynamic therapy | 5 |
| Stent placement | 5–25 |
Spontaneous perforation of the oesophagus
Boerhaave’s syndrome is characterised by barogenic oesophageal injury leading to immediate and gross gastric content contamination of the pleural cavity. However, various degrees of damage and contamination are possible. As a result, a number of clinical terms have evolved to describe these events: this text will only use the term ‘spontaneous perforation of the oesophagus’, with the term ‘disruption’ used to describe the ‘process’ of perforation. Spontaneous perforation of the oesophagus is most accurately defined as complete disruption of the oesophageal wall occurring in the absence of pre-existing pathology. Since the oesophagus possesses no serosa, transgression of oesophagogastric contents leads rapidly to chemical and septic mediastinitis. In 80–90% of cases, this disruption is associated with a sudden rise in intra-abdominal pressure, most usually as a result of retching or vomiting; however, blunt trauma, weightlifting, parturition, defecation, the Heimlich manoeuvre or status epilepticus have all been cited as causal factors. Although vomiting is commonplace, spontaneous oesophageal perforation is not, which suggests that other as yet unidentified factors may be important, such as pre-existing anatomical or pathological abnormalities. However, an underlying pathology is identified in only 10–20% of cases, such as malignancy, peptic ulceration or infection (as such not truly spontaneous perforation). A common misconception is that Mallory–Weiss tears represent part of the spectrum of spontaneous perforation but it is likely that these mucosal injuries reflect ‘shearing’ rather than ‘barogenic’ trauma.3 Equally, eosinophilic oesophagitis has also been associated with an increased risk of both mucosal tears and full-thickness perforation either spontaneously induced by vomiting to dislodge impacted food or following endoscopic procedures.4
Clinical presentation
As a result, in spontaneous perforation, the diagnostic error is high, with only 5% of cases diagnosed at presentation. This leads to diagnostic delay of greater than 12 hours in the majority of cases.7 It may be that less than 35% of cases are correctly diagnosed pre-mortem8 (Box 19.1). As time passes, the critical condition of the patient further obscures relevant clinical features and the pursuit of incorrect investigations makes the diagnosis even more elusive.
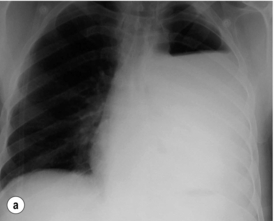
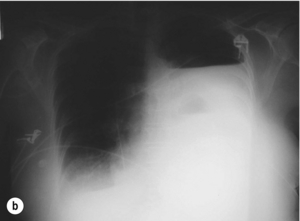
Depending on the aetiology and amount of contamination, pain may be severe, constant, retrosternal or epigastric, distressing, exacerbated by movement and poorly relieved by narcotics or relatively mild. Dysphagia and odynophagia are common. Patients can be tachypnoeic and may sit up to splint their diaphragm. Abdominal pain or tenderness are not uncommon and can lead to a negative laparotomy.6 Similarly, subcutaneous emphysema takes time to develop; mediastinal emphysema precedes this and may be visible on a plain chest radiograph. With time the negative intrathoracic pressure draws air, food and fluids into the mediastinum and pleural cavities and a chemical pleuromediastinitis develops. A low-grade pyrexia ensues, and a sympathetic nervous system response develops with pallor, sweating, peripheral circulatory shutdown, tachycardia, tachypnoea and overt haemodynamic shock, which worsens as the systemic inflammatory response gives way to sepsis. Within 24–48 hours cardiopulmonary embarrassment and collapse develop as a consequence of overwhelming bacterial mediastinitis and septic shock. The combination of chest pain and shock may inappropriately, but all too commonly lead to a cardiological referral. Survival is dependent on the evacuation of the contamination, from the mediastinal and pleural cavities at the earliest possible opportunity.9 Systemic effects are less common when the cervical oesophagus is damaged, with neck pain, torticollis, dysphonia, cervical dysphagia, hoarseness and subcutaneous emphysema predominating.
Investigations
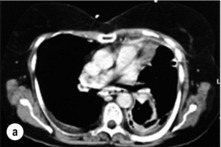
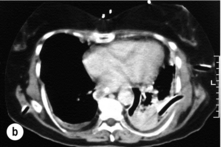
The typical findings on plain chest radiography are subtle – dependent on the site and the time interval following the insult. These are documented in Box 19.2 and Fig. 19.1. A plain abdominal radiograph may help to exclude a perforated intra-abdominal viscus.7
Contrast radiography
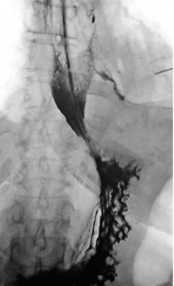
Oral water-soluble contrast radiography ascertains the site, the degree of containment and the degree of drainage of the perforation (Fig. 19.2). Aqueous agents are rapidly absorbed, do not exacerbate inflammation and have minimal tissue effects. However, false-negative results in 27–66% and the limited applicability to a collapsed, unwell patient have downgraded their usefulness.
Upper gastrointestinal endoscopy
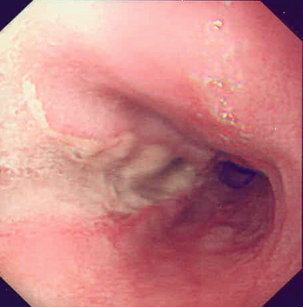
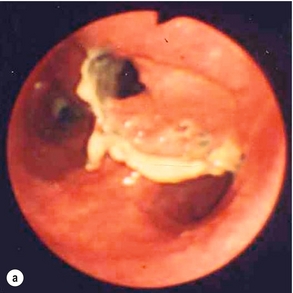
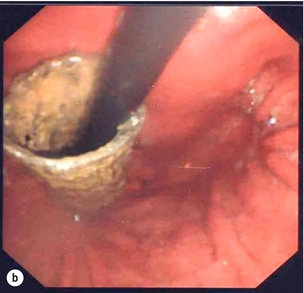
Endoscopic assessment excludes the diagnosis if normal, influences management if underlying pathology is discovered and facilitates the placement of a nasojejunal tube to allow enteral feeding. Risks are minimised using modern, flexible videoscopes together with fluoroscopic guidance, but should only be performed by a highly experienced endoscopist conversant with the consequences of their actions (Figs 19.3 and 19.4). Endoscopy can be performed in the sickest of patients, if necessary ‘on table’, when other injuries or instability of the patient preclude radiological assessment.
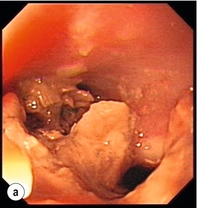
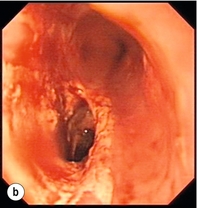
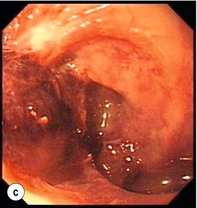
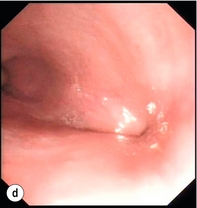
Figure 19.4 Endoscopic appearances of iatrogenic perforation. (a) Food bolus with false iatrogenic lumen alongside. (b) Appearance after food bolus removed. (c) Contained mediastinal cavity. (d) Six weeks later following conservative management a small pit remains.
Management
All patients with an oesophageal perforation are critically ill. The immediate priorities are the establishment of a secure and adequate airway, stabilisation of cardiovascular status and relief of pain, often using opiate-based analgesia. Regular reassessment is obligatory as an initially stable patient can rapidly decompensate. An early anaesthetic review is recommended. Box 19.3 documents the initial resuscitation.
Non-operative management
Non-operative management, endoscopic and minimally invasive operative management have all been shown to be safe and feasible in carefully selected patients who have either been diagnosed with minimal contamination and no mediastinitis or with a contained perforation. It may also be considered in those with a delayed diagnosis who have demonstrated tolerance.9
Criteria have been developed to aid the selection of suitable patients for non-operative management. These are detailed in Box 19.4. Case series applying these criteria demonstrate a mortality rate between zero and 16%, but numbers are small and results are skewed by both selection and publication bias.
Adjuncts to non-operative management
Closure: clips and sealants: Endoclips are well established in closing small, clean defects after endoscopic mucosal resection or submucosal dissection for early cancer.14,15 In the absence of significant contamination, small iatrogenic perforations may be closed immediately using endoclips in addition to supportive non-operative treatment. However, endoclipping ‘en face’ in the oesophagus is extremely challenging and should only be attempted by highly skilled endoscopists. It is also debatable whether this significantly alters the clinical course over a simple non-operative approach.2 There is at least one case report of clipping a spontaneous oesophageal perforation but this cannot be recommended in the face of gross contamination.16
Diversion: stents: Self-expanding stents have been used to seal oesophageal perforations, chronic fistulas and even postoperative anastomotic leaks.17–19 Stents were not designed for use in a normal oesophagus and migration rates approach 30%, and concerns have been raised in terms of extending the defect through pressure necrosis and through the trauma of their subsequent removal.14,20 Publication bias means that failure and the consequences of failure remain unknown. There is considerable variation in the timing of stent placement and number of stents used. It is evident that the majority of cases also involve aggressive non-operative management.21–24 It is therefore difficult to attribute successful outcomes to the stent placement alone. For example, one ‘successful’ report documents a patient who had five stents placed over an 8-month period before eventually proceeding to oesophagectomy at a tertiary referral centre.21 The one prospective stenting study lists 10 patients with a Boerhaave perforation.25 Stent migration was high (11 out of 33) and there was a 50% complication rate (bleeding/stent fracture/impacted stent) if stents were not removed before 6 weeks.
At present there is insufficient evidence to support the use of oesophageal stents in oesophageal perforations. The authors suggest that their use is highly selective and should always be viewed as a temporary solution. However, in patients whose physical condition precludes more aggressive treatments and those in whom resection is not deemed suitable, stents do offer a serious alternative. If utilised then the stent should be removed within 3 months to avoid long-term complications since the biggest concern is septic erosion into surrounding structures. This horrendous situation does not appear to be represented in the literature (Fig. 19.6).25
Drainage: repeated endoscopy: Endoscopic lavage and drainage of contained mediastinal perforations or even endoscopic placement of a vacuum sponge drainage system has been used for liquid contamination. This is certainly a novel approach but labour intensive and not suitable for gross contamination. Success may again simply reflect patients who would have done well with more simple non-operative treatment.26,27
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree