Fig. 14.1
Mechanism of injuries resulting from external ocular compression and resulting reperfusion injuries
Other rare causes of CRAO include embolism to the retinal circulation, decreased blood flow secondary to systemic hypoperfusion, impaired venous drainage of the retina or coagulation disorder [18].
Signs and symptoms of patients with postoperative CRAO include painless unilateral visual loss, no light perception, afferent pupil defect, periorbital oedema, chemosis, proptosis, ptosis paraesthesia of the supraorbital region and corneal abrasion [19]. Diagnosis is prompted by the sudden onset of visual loss and the presence of retinal whitening with or without classical ‘cherry-red’ macula on fundoscopy (Fig. 14.2).


Fig. 14.2
Fundus photography of the right eye with non-arteritic CRAO demonstrating cherry-red spot and retinal opacity of the posterior fundus (Reprinted from Hayreh, Sohan Singh. Ocular Vascular Occlusive Disorders. © Springer International Publishing, Switzerland 2015. Chapter 13, Central Retinal Artery Occlusion; p. 239. With permission of Springer Nature)
Prognosis for CRAO is generally poor and treatment inadequate. Cold compress, ocular massage and vasodilatation via induced hypercapnia have been advocated in presentations less than 90 min. Paracentesis may facilitate distal migration of the embolus limiting extent of injury. Fastidious attention to patient positioning aimed at avoiding external ocular pressure is paramount in prevention of CRAO.
Branch retinal artery occlusion (BRAO) causes permanent ischaemic retinal damage with partial visual field loss. BRAO is primarily the result of emboli. The vast majority of reported cases are associated with cardiopulmonary bypass where circulating embolic material is implicated. Embolism passage from surgical site via the venous system and a patent foramen ovale has been reported as a cause of perioperative retinal vascular occlusion in spinal surgery [20]. BRAO is associated with painless partial visual field loss and sectoral whitening in the path of a branch retinal artery on fundoscopy.
Ischaemic Optic Neuropathy
Ischaemic optic neuropathy (ION) refers to ischaemic damage to the optic nerve itself. ION is subclassified into arteritic or non-arteritic ION. Arteritic ION is secondary to inflammation of blood vessels chiefly associated with giant cell/temporal arteritis and responds to steroid therapy. In contrast, non-arteritic ION is secondary to occlusive disease or other noninflammatory disorders. In the general population, non-arteritic ION is the leading cause of sudden visual loss in patients above 50 years of age with an annual incidence in the United States of 82 per 100,000 persons [21]. Non-arteritic ION is the overwhelming cause of POVL. It has been reported after a wide spectrum of surgical procedures, most commonly cardiothoracic surgery [18], instrumented spinal fusion [22] and head and neck surgery [23, 24]. Multiple cases following gynaecological, urological and general surgical procedures have also been reported [25].
ION is further classified by the location of the nerve ischaemia into anterior ischaemic optical neuropathy (AION) and posterior ischaemic optic neuropathy (PION) . This classification is of importance due to the difference in incidences, proposed aetiologies and clinical presentations of each group. Postoperative AION predominately occurs following cardiothoracic surgeries. All reported cases of POVL secondary to ION related to robotic pelvic surgery have been PION injuries [1, 25]. Similarly the vast majority of reported ION following spinal surgery have been posterior injuries.
The exact mechanism of PION and AION is contentious and likely multifactorial. Posterior ischaemia occurs behind the globe and is probably not related to predictable increases in intraocular pressure; it may well be related to disruption of blood supply to the optic nerve from a network of very small perforating pial arteries (Fig. 14.3). In contrast AION proposed to be caused by disruption of blood supply through the posterior ciliary arteries feeding the head of the optic nerve, and this condition may be related to impaired autoregulation of flow (perfusion pressure vs intraocular pressure).
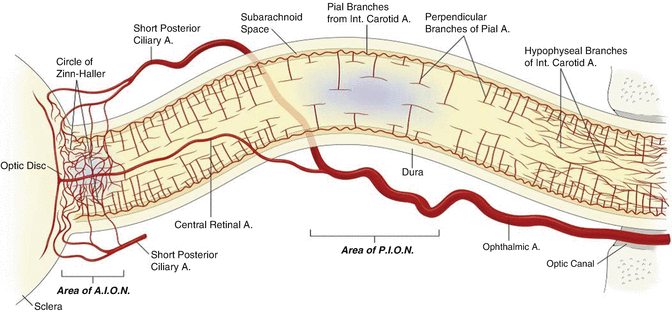

Fig. 14.3
Diagram of the orbital optic nerve and arterial supply. Areas implicated in ischaemic optic neuropathy are indicated in blue. The mid-orbital optical nerve has a paucity of blood supply compared to the anterior component. This area supplied by only the pial branches is the region involved in PION. The pial branches have variable density and in an unusual perpendicular T-shaped pattern, characteristic of a low pressure system. There is low density of arteriolar and capillary supply to this mid-orbital segment compared with the canalicular or retrobulbar segments of the optic nerve. Abbreviations: A artery, AION anterior ischaemic optic neuropathy, PION posterior ischaemic optic neuropathy
AION and PION have been reported in the setting of massive fluid replacement especially in prone-positioned patients. Excessive fluid administration could result in increased IOP or accumulation of fluid in the optic nerve or both. As the retinal vein exits out of the optic nerve, the oedematous nerve may inhibit venous outflow resulting in an internal ‘compartment syndrome ’ [4]. Patients on the ASA Postoperative Visual Loss Registry received on average 9.7 L of crystalloids intraoperatively, suggesting that fluid replacement may play a role [26].
Key surgical factors linked to perioperative ION are prolonged prone or steep Trendelenburg positioning, prolonged overall surgical duration and massive blood loss. Possible intraoperative haemodynamic factors include decreased systemic blood pressure, anaemia or haemodilution, a high ratio of crystalloid to colloid fluid replacement and venous congestion. Characteristics of the optic nerve and disc may predispose to ION such as reduced flow of cerebrospinal fluid, abnormal auto regulation, anatomic variants in blood supply and small cup-to-disc ratio. Potential systemic risk factors include hypertension, diabetes, atherosclerosis, hyperlipidaemia, smoking history and hypercoagulability [4, 14, 18, 26, 27]. Minimization of these potential risk factors where possible is the basis of ION prevention.
Typically PION results in complete visual loss within 24 h postoperatively compared to AION where two thirds of cases were not evident until more than 24 h following surgery and initial symptoms more likely to be incomplete visual loss. Bilateral visual loss is more common with PION (63%) compared with AION (52%). Nearly all patients with AION have disc oedema, pallor or both on initial assessment (Fig. 14.4). In comparison PION is associated with a normal optic disc on initial fundoscopic evaluation in 92% of patients [4, 14].


Fig. 14.4
Fundoscopy in acute non-arteritic anterior ischaemic optic neuropathy. The optic disc is oedematous and hyperaemic. Splinter haemorrhages (Arrow) are present
No effective treatment for ION has been proven. Only approximately 30% of patients with either AION or PION will have any improvement. The focus of management is therefore on prevention.
Cortical Blindness
Cortical blindness is the result of decreased perfusion to the occipital cortex by tributaries of the posterior cerebral artery. The cause is either hypoperfusion or embolic phenomenon. Cortical blindness is a very rare cause of POVL that is usually associated with cardiac surgery [4, 28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







