CHAPTER 6 Obesity
Obesity is a chronic disease that is increasing in prevalence. Both the World Health Organization1 and the National Heart, Lung, and Blood Institute2 of the U.S. National Institutes of Health have labeled obesity an epidemic. More than 30% of adult Americans are now obese3 and the prevalence of obesity in children and adults has increased more than 50% in the past decade. The progress of this epidemic is shown in maps indicating obesity rates in all states and regions of the United States. In 1991, 18% of the states (9/50) had obesity rates exceeding 15%, and by 1998 this rate had increased to 78% (39/50; Fig. 6-1). This epidemic is a time bomb for the future development of diabetes and its many complications.4
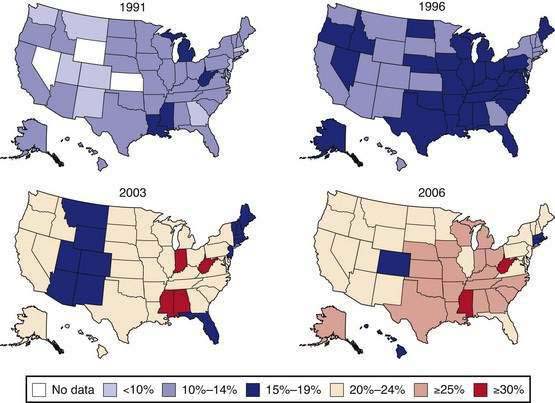
Data of the Behavioral Risk Factor Surveillance System. (From the Centers for Disease Control and Prevention. U.S. obesity trends 1985-2007. Available at http://www.cdc.gov/nccdphp/dnpa/obesity/trend/maps/index.htm.)
As a disease, obesity has its pathology rooted in the enlargement of fat cells, the secretory products of which produce most of the pathogenic changes that result in the complications associated with obesity. The remaining changes are a consequence of the fat mass per se.4 Physicians and the health care system have two strategies to deal with this problem. First, we can prevent the development of obesity, or treat it before complications develop. Alternatively, we can wait until complications develop and then treat these. Many physicians would prefer to wait until the comorbidities arise, because there are more treatment options for diabetes, hypertension, and heart disease than for obesity. In one long-term trial, the incidence of new cases of diabetes was reduced to zero over two years in patients who lost weight and maintained a weight loss of 12% or more, compared with an incidence of 8.5% for new cases of diabetes in those who did not lose weight.5
Obesity is a stigmatized disease.4 A common view shared by the public and by health professionals alike is that obese people are lazy and weak-willed. It is not unusual to hear statements such as, “If fat people just had will power, they would push themselves away from the table and not be so fat.” This view is supported by the perception in advertising that thin women are more attractive than full-figured women; the declining relative weights of center-fold models in Playboy and other publications and of women who are winners of the Miss America contest substantiate this view.
Another issue that aggravates the problem of treating obesity is the negative perception that surrounds the use of appetite suppressants.4 Amphetamine, the first widely used weight loss drug, is addictive. This concern about addiction has been transferred to other drugs, whether warranted or not.
With all treatments for obesity, weight loss slows and then stops. This so-called plateau effect arises when homeostatic mechanisms in the body come into play and stabilize weight, although at a lower level than the starting level. A similar phenomenon also is observed in the treatment of hypertension; when an antihypertensive drug produces a drop in blood pressure, there is a plateau at a new, lower level.3 The antihypertensive drug has not lost its effect when the plateau occurs, but its effect is being counteracted by physiologic mechanisms. In the treatment of obesity, this new plateau in body weight often is viewed as a therapeutic failure for the weight loss drug or other treatment. Cessation of weight loss often prompts patients to think that they are “cured” and they stop treatment only to regain weight.
Finally, many treatments for obesity have provided unwanted side effects.4 A recent example is what befell many patients who took the combination of fenfluramine and phentermine. The aortic regurgitant lesions that occurred in up to 25% of the patients treated with this combination of drugs led many physicians to say “I told you so” and “I’m certainly glad I didn’t use those drugs.” Much of this suspicion will subside with time, but there will remain residual concerns about such potential side effects of antiobesity agents among physicians, government regulators, and the public.
DEFINITION
BODY MASS INDEX
Throughout the past 50 years, there has been a steady rightward, upward shift in the distribution curve for body weight. This trend can be traced most effectively using the body mass index (BMI), defined as the weight in kilograms divided by the height (in meters) squared [W/(H)2], which provides a useful operating definition of overweight. A normal BMI is between 18.5 and 25 kg/m2. A BMI between 25 and 29.9 kg/m2 is operationally defined as overweight, and individuals with a BMI higher than 30 kg/m2 are obese, with special consideration for muscle builders and other resistance-trained athletes. Above a BMI of 30 kg/m2, BMI is specific, but not very sensitive when compared with body fat for determining obesity.6 Values below a BMI of 30 kg/m2 are less specific, but still represents a good starting point in evaluating overweight people. BMI also provides a good risk measure for obesity.1,2,4
CENTRAL ADIPOSITY
The waist circumference is a practical measure of central adiposity that is a surrogate for more precise measures of visceral fat, such as computed tomography (CT) or magnetic resonance imaging (MRI) scans of the abdomen. When BMI and waist circumference were used to predict the risk of hypertension, dyslipidemia, and the metabolic syndrome, the waist circumference was shown to be a better predictor than the BMI.7
PREVALENCE AND COSTS
Using the BMI, it is clear that obesity is increasing in prevalence. This increase began in the 1980s and continues at present, although recent data suggest that it may be abating somewhat.3 Obesity affects children as well as adults. We are now seeing a rise in the prevalence of type 2 diabetes in adolescents that is directly related to the rising prevalence of obesity. Obesity has a higher prevalence in Hispanic and African American populations.8,9 Both mean height and weight increased in adults ages 20 to 74 years between 1960 and 2002. Men increased in height from a mean of 68 inches (172.7 cm) to 69.5 inches (176.5 cm) and women from a mean of 63 to 64 inches (160 cm to 162.6 cm) during this period. For men, weight rose from a mean of 166.3 to 191 pounds (75.4 to 86.8 kg) and for women from a mean 140.2 to 164.3 pounds (63.7 to 74.6 kg), for an average increase of BMI from 25.2 to 28 kg/m2 for men and from 24.8 to 28.2 kg/m2 for women during this 42-year period. The increase in weight was greater in older men than in younger men, but the reverse was true for women, with older women gaining less weight than younger women. Similar effects are seen in children, with the weight of 10-year-old boys rising from a mean of 74.2 pounds (33.7 kg) in 1963 to 85 pounds (38.6 kg) in 2002, and of 10-year-old girls rising from a mean of 77.4 pounds (35.2 kg) to 88 pounds (40 kg) in this same interval. These increases in weight were associated with increases in BMI for boys and girls. For 7-year-old boys, BMI increased from a mean of 15.8 to 17.0 kg/m2 between 1963 and 2002 and for 7-year-old girls it rose from a mean of 15.8 to 16.6 kg/m2. For 16-year-old boys, it rose from 21.3 to 24.1 kg/m2 and for girls, from 21.9 to 24.0 kg/m2 in this same interval.
Obesity is expensive, representing between 3% and 8% of health care budgets in various countries.4,10 Hospital costs and use of medication also escalate with increasing BMI. In a large health maintenance organization, mean annual costs were 25% higher in participants with a BMI between 30 and 35 kg/m2 and 44% higher in those with a BMI greater than 35 kg/m2 than in those with a BMI between 20 and 25 kg/m2.11 Costs for lifetime treatment of hypertension, hypercholesterolemia, type 2 diabetes, heart disease, and stroke in men and women with a BMI of 37.5 kg/m2 was $10,000 higher than for men and women with a BMI of 22.5 kg/m2, according to data from the National Center for Health Statistics and the Framingham Heart Study.12
CAUSES
ENERGY IMBALANCE: EPIDEMIOLOGIC MODEL
We become obese because over an extended period of time, we ingest more carbon- and nitrogen-containing compounds as food than we need for daily energy expenditure. We and other animals thus obey the First Law of Thermodynamics, which describes this energy imbalance. Unfortunately, however, this important law of nature fails to inform us about such important issues as how food intake is regulated, where fat is stored, why men store fat differently than women, and how genes control these processes.4,13
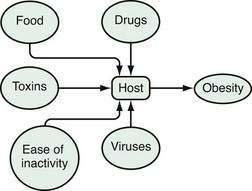
An epidemiologic model is a good way to look at energy balance and to conceptualize obesity as a disease.14 In an epidemiologic model, environmental agents act on a host to produce a disease (Fig. 6-2). Disease is a function of the virulence of the agent and susceptibility of the host. For obesity, the environmental agents include drugs, food, toxins, physical inactivity, viruses, and other people.4,13,15 Among the important drugs that cause weight gains are some antipsychotic drugs (e.g., clozapine, olanzapine), some antidepressants (e.g., amitriptyline), some antidiabetic drugs (insulin, sulfonylureas, and thiazolidinediones), and glucocorticoids (Table 6-1). Although neurotropic viruses can produce obesity in experimental animals, the most intriguing human example is the presence of antibodies to adenovirus 36 in some obese people, and the fact that this virus can produce obesity in marmosets, a nonhuman primate. Exposure to estrogen-like compounds in utero may enhance the risk of obesity later in life. In affluent Western societies, foods, particularly foods high in fat, are abundant. In addition, portion sizes have increased, providing more energy to people with each portion. Toxins are an interesting potential group of agents for which more research is needed. Viruses are known to produce obesity and their potential role in obesity needs to be studied further. Physical activity may have gradually decreased, thereby reducing energy expenditure. Some have described the current environment as a virulent or toxic environment that has heightened the risk for obesity for people who are genetically susceptible to becoming obese.15 For the genetically susceptible host, this excess of food energy, environmental toxins, and viruses, along with a reduced level of physical activity, may lead to an accumulation of fat in fat cells.
Table 6-1 Drugs That Produce Weight Gain and Potential Alternatives
| CATEGORY | DRUGS THAT CAUSE WEIGHT GAIN | POTENTIAL ALTERNATIVES |
|---|---|---|
| Neuroleptics | Thioridazine, olanzepine, quetiapine, resperidone, clozapine | Molindone, haloperidol, ziprasodone |
| Antidepressants | ||
| Tricyclics | Amitriptyline, nortriptyline | Protriptyline |
| Monoamine oxidase inhibitors | Imipramine, mitrazapine | Bupropion, nefazadone |
| Selective serotonin-reuptake inhibitors | Paroxetine | Fluoxetine, sertraline |
| Anticonvulsants | Valproate, carbamazepine, gabapentin | Topiramate, lamotrigine, zonisamide |
| Antidiabetic drugs | Insulin, sulfonylureas, thiazolidinediones | Acarbose, miglitol, metformin, pramlintide exenatide |
| Antiserotonin agents | Pizotifen | — |
| Antihistamines | Cyproheptadine | Inhalers, decongestants |
| Steroid hormones | Contraceptives, glucocorticoids, progestational steroids | Barrier methods, nonsteroidal anti-inflammatory drugs |
ENVIRONMENTAL AGENTS
Intrauterine Factors
Several environmental intrauterine events influence postnatal weight and lifetime weight gain and fatness.4 These include maternal diabetes, maternal smoking,16 and intrauterine nutrition, all of which increase an individual’s risk for increased body weight and diabetes later in life. Offspring of mothers who smoke during pregnancy are at increased risk of weight gain in their first decades of life,16 as are infants of diabetic mothers and small-for-gestational-age infants.
Neonatal Environment
Infants who are breast-fed for more than three months may have a reduced risk of future obesity.17 Children who get more sleep tend to weigh less when they enter school than those who sleep less.4
Adiposity Rebound
Adiposity rebound occurs in childhood at the age that the BMI stops falling and begins to rise. Early adiposity rebound predicts future obesity.18
Drug-Induced Weight Gain
In our current practice of medicating much of society, it would not be surprising to find drugs that produce weight gain. Table 6-1 is a list of medications that produce weight gain when used to treat other diseases, such as psychosis, depression, allergies, and diabetes. Also listed in the table are possible alternatives that can be used to avoid such weight gain. In most cases, there are alternative strategies that can be used to treat a patient when weight gain is closely associated with the initiation of a new medication for one of these conditions. When the binding of drugs listed in Table 6-1 to receptors in the brain was examined, several—including the histamine (H1), α1A-adrenergic, and serotonin (5-HT2C and 5-HT6) receptors—could explain much of the differences in weight gain associated with the atypical antipsychotic drugs shown in Table 6-1.19
Diet
Portion size, fat intake, and consumption of beverages sweetened with sucrose (table sugar) or high-fructose corn syrup have all been implicated in the current obesity epidemic.4,20 Consumption of soft drinks predicted future weight gain in children and adults, and also may be related to the risk for the metabolic syndrome and gout.21
Physical Inactivity
Low levels of physical activity, such as watching television, correlate with weight gain. In a 10-year study of individuals 20 to 74 years of age in the National Health and Examination Survey (NHANES I), those with low levels of recreational activity gained more weight than those with higher levels of activity.22 Similarly, low levels of baseline energy expenditure predicted weight gain in the Pima Indians,23 and exercise capacity and body composition predict mortality among men with diabetes.24 Time spent watching television correlates with percentage of overweight children4 and the more television watched, the greater the risk of overweight and obesity.
Smoking
Smokers have lower body weights than nonsmokers, and cessation of smoking generally is associated with weight gain.4,25 Two explanations have been offered for the effect of smoking on body weight. First, smoking is thermogenic; that is, the metabolic rate during the act of smoking is higher than when the subject is not smoking. Second, smoking reduces hunger and changes taste perceptions; smokers tend to eat less.
Viruses
One laboratory has reported that obese humans have higher antibody levels to one strain of adenovirus (AM-36).26 As noted, this virus can produce obesity when given to nonhuman primates and this viral antibody, as a marker of viral infection, is found in the circulation of many obese people.
HOST AGENTS
Genetic Causes
Several genes have a strong relation to the development of obesity.27 The melanocortin-4 receptor gene, leptin gene, pro-opiomelanocortin (POMC) gene, and agouti gene all have significant effects on body fat and fat stores. There are five melanocortin receptors, MC4 and MC being primarily in areas of the brain that affect feeding. Genetic abnormalities in this receptor may account for up to 6% of cases in early-onset, severely obese children.28 Absence of leptin or an ineffective leptin receptor is associated with massive obesity in human beings and animals. Leptin has the dual effect of reducing food intake and increasing energy expenditure, both of which favor loss of body fat. Treatment of leptin-deficient children with leptin decreased their body weight and hunger, indicating the importance of leptin in normal subjects (see later, “Neurophysiologic Factors”). Heterozygotes for leptin deficiency have low but detectable serum leptin levels and have increased adiposity, indicating that low levels of leptin are associated with increased hunger and gain in body fat. Leptin also can increase energy expenditure. Thus, when leptin is given and caloric intake simultaneously is reduced, the leptin attenuates the decreases in thyroid hormones and 24-hour energy expenditure that result, because leptin normally falls with reduced caloric intake.29
There are several rare clinical syndromes of obesity with a genetic basis.30 The Prader-Willi syndrome is the most common.31 This disease is transmitted as an abnormality on chromosome 15 and is characterized by a floppy baby who has difficulty feeding. These children are mentally slow, short in stature, have hypotonia and hypogonadism, and are obese. The Bardet-Biedl syndrome can result from changes at many different genetic loci. These children have visual impairment caused by abnormalities in the retina, are mentally slow, and have increased numbers of digits (polydactylism). In at least one pedigree, the genetic defect was caused by a fault in the chaperonin-like gene, which produces a product involved in folding proteins in the reticular endoplasm.32
The epidemic of obesity is occurring on a genetic background that does not change as fast as the epidemic has been exploding. Nonetheless, it is clear that genetic factors play an important role in the development of obesity, for which more than 100 genes have so far been implicated.30
Neurophysiologic Factors
A number of peptides play an important role in the development of obesity.33 The discovery of leptin in 1994 opened a new window for understanding control of food intake and body weight. The response of leptin-deficient children to treatment with leptin revealed the critical role that this peptide plays in the control of energy balance.27 Leptin enters brain tissue, probably by transport across the blood-brain barrier, where it acts on receptors in the arcuate nucleus to regulate, in a conjugate fashion, the production and release of at least four peptides.34 Leptin inhibits the production of neuropeptide Y (NPY) and agouti-related peptide (AGRP), both of which increase food intake; it also enhances production of POMC, the source of α-melanocyte stimulating hormone (α-MSH), which reduces food intake.
Three other brain peptide systems also have been linked to the control of feeding. Melanin-concentrating hormone (MCH) is found in the lateral hypothalamus and decreases food intake when injected into the ventricular system of the brain.35 Orexin (also called hypocretin) was identified in a search for G protein-linked peptides that affect food intake34; it increases food intake and plays a role in sleep. Endocannabinoids (anandamide and 2-arachidonoyl glycerol) also increase food intake by acting on cannabinoid-1 (CB1) receptors. There are two cannabinoid receptors that originally were identified when tetrahydrocannabinol, the active ingredient of marijuana, was used to identify its endogenous receptor. These receptors are located in the brain and in many peripheral tissues. An antagonist to the CB1 receptor has served as the basis for a new antiobesity drug.36
Intestinal peptides, including cholecystokinin, pancreatic polypeptide, and polypeptide YY, reduce food intake,4 whereas ghrelin, a small peptide produced in the stomach, stimulates food intake.33
Metabolism of fatty acids in the brain may be another important control point. An experimental chemical that blocks fatty acid synthase in the brain was noted to result in significant weight loss and accumulation of malonyl coenzyme A. In pursuing this system, 5′-adenosine monophosphate kinase (AMPK), an enzyme that is activated or inhibited in relation to the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), was thought perhaps to be the underlying central point in this control system.37
PATHOLOGY AND PATHOPHYSIOLOGY
The pathology and pathophysiology of obesity lie in the changes in the fat cells that store fat. Enlarged fat cells are the hallmark of this process. These enlarged cells produce effects through their increased mass, which increases the wear and tear on joints and makes overweight individuals obvious candidates for stigmatization. In addition, enlarged fat cells produce many adipokine products that affect distant cells. In addition to enlarged fat cells, some individuals also have an increased number of fat cells.14,38
FAT CELL AS AN ENDOCRINE CELL
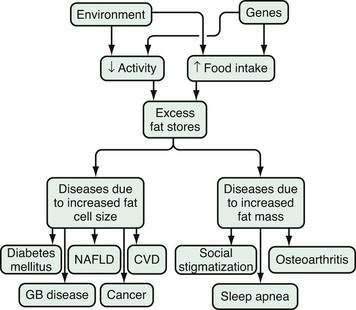
Two mechanisms are integral to understand the pathophysiology of health problems associated with obesity. The first is the increased fat mass, which explains the stigmatization of physically obvious obesity and accompanying osteoarthritis and sleep apnea (Fig. 6-3).14 The second is the consequences of the increased number of peptides produced by the enlarged fat cells that act on distant organs. The discovery of leptin catapulted the fat cell into the arena of endocrine cells. In addition to leptin, fat cells secrete, for example, cytokines, angiotensinogen, adipsin (complement D), and metabolites, such as free fatty acids and lactate. Angiotensinogen is an enzyme involved in the control of blood pressure. Adipsin was one of the first substances secreted by fat cells to be identified, and turned out to be a component of the coagulation system. In contrast to these fat cell products, the release of adiponectin, the most abundant peptide produced by fat cells, is decreased in obesity.39 High levels of adiponectin are associated with insulin sensitivity and low levels with insulin resistance. These products of the fat cell in turn modify the metabolic processes of other organs in the host. For the susceptible host, these metabolic changes lead in turn to hyperinsulinemia, atherosclerosis, hypertension, and physical stress on bones and joints.
VISCERAL FAT
A considerable body of data suggests that visceral fat has a stronger relationship with the complications associated with obesity than does total body fat.7 Moreover, central adiposity is one of the key components of the metabolic syndrome, the diagnostic criteria of which are based on the recommendations of the National Cholesterol Education Program Adult Treatment Panel III (Table 6-2).40,41
Table 6-2 National Cholesterol Education Program Adult Treatment Panel (ATP) III Modified Criteria for Metabolic Syndrome*
| PARAMETER | ATP III MODIFIED CRITERION |
|---|---|
| Waist circumference | |
| Female | >35 inches (>88 cm) |
| Male | >40 inches (>102 cm) |
| HDL cholesterol | |
| Female | <50 mg/dL (<1.29 mmol/L) |
| Male | <40 mg/dL (<1.03 mmol/L) |
| Fasting glucose | ≥110 mg/dL (≥6.2 mmol/L) |
| Triglycerides | ≥150 mg/dL |
| Blood pressure | ≥130/85 mm Hg |
HDL, high-density lipoprotein.
* The metabolic syndrome is present when any three of the five criteria listed in the table are abnormal.
Adapted from Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486-97; and Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: Prevalence and associated risk factor findings in the U.S. population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003; 163:427-36.
COMPLICATIONS AND CONSEQUENCES
DEATH
Mortality and BMI have a J-shaped relationship in essentially all studies.4 Among the more than 90,000 women in the Women’s Health Initiative, there was a graded increase in the risk of death as BMI increased from normal to a BMI > 40 kg/m2.42 In the more than 527,000 men and women in the NIH-AARP American cohort of individuals ages 50 to 71 years, the risk of death was increased both in those who were overweight and in those who were obese.43 In an even larger Korean study, both overweight and obesity in men and women were related to higher death rates compared with normal-weight subjects.44 Another American cohort of more than 80,000 men and women was monitored for over 14.7 years and over 1.23 million person-years of follow-up. Excluding the first five years of death in men and women, those younger than 55 years showed a risk of death that was directly related to BMI, beginning at a BMI of 21.0 kg/m2 in women and 23.0 kg/m2 in men; in those older than 55 years, the increase in mortality occurred at a higher BMI (25 kg/m2 in women and 30 kg/m2 in men).45
DISEASES AND DISORDERS
Disorders Related to Enlarged and Visceral Fat Cells
Excess body fat, particularly visceral fat, increases the risk for a number of diseases as a metabolic consequence of the enlarged fat cells or as a result of the increased mass of fat.4,14,46
Diabetes
The risk of diabetes rises as BMI increases and is particularly steep when the BMI is more than 30 kg/m2. Weight gain in middle age, independent of attained weight, increases the risk of impaired glucose tolerance45 and increases the risk of heart disease. Blood pressure increases linearly with BMI and hypertension is present in approximately half of very obese subjects at initial evaluation.47 In the Nurses Health Study, the BMI values at age 18 years and at midlife were positively associated with the occurrence of hypertension.4
Lipid Derangements
Dyslipidemia, characterized by a low high-density lipoprotein (HDL) cholesterol and high triglyceride level, is more common in obesity, particularly with central adiposity48 and, when accompanied by hypertension and an elevated serum glucose level, meet the National Cholesterol Education Program criteria for the so-called metabolic syndrome. A meta-analysis of 21 cohort studies has suggested that the adverse effects of obesity on blood pressure and lipids account for approximately half of the excess risk of coronary heart disease.49
Cardiovascular Diseases
Because coronary heart disease accounts for nearly half of all deaths in our society, its relationship to obesity is particularly important.50 In one study, an increase in BMI of 1.1 kg/m2 increased the risk for major cardiovascular disease by 6%.51 Obesity also increases the risk of congestive heart failure52 and atrial fibrillation.53 Much of this increased risk of heart disease is associated with central adiposity.7 The INTERHEART study of patients from 52 countries showed that abdominal adiposity accounts for 20% of the population’s attributable risk for a first myocardial infarction.54
Hypertension
Blood pressure often is increased in overweight individuals.55 In the Swedish Obese Subjects Study,48 hypertension was present at baseline in 44% to 51% of subjects. For each decline of 1 mm Hg in diastolic blood pressure, the risk of myocardial infarction decreased an estimated 2% to 3%. Obesity and hypertension interact with cardiac function. In overweight individuals, ventricular eccentric dilation occurs, whereas hypertension in normal-weight people produces concentric hypertrophy of the heart, with uniform thickening of ventricular walls. Increased preload and stroke work are associated with hypertension. The combination of overweight and hypertension leads to thickening of the ventricular wall and larger heart volume, and thus to a greater likelihood of cardiac failure.
Kidney Disease
Obesity may affect the kidney in several ways. First, an obesity-related glomerulopathy characterized as focal segmental glomerulosclerosis has increased significantly, from 0.2% of biopsies collected between 1986 and 1990 to 2.0% of specimens taken between 1996 and 2000.56 Second, overweight patients also are at increased risk for kidney stones.57 Finally, BMI is related to the risk of end-stage renal disease. In a study from the Kaiser Permanente Group of Northern California, Hsu and colleagues58 found that a higher BMI is a progressively greater risk factor for end-stage renal disease that persists even after correcting for multiple potential confounding factors, including baseline blood pressure or diabetes mellitus.
Gallbladder Disease
Cholelithiasis is the primary hepatobiliary pathology associated with overweight,59 part of the explanation for which is increased cholesterol turnover related to total body fat.60 Cholesterol production is linearly related to body fat; approximately 20 mg of additional cholesterol is synthesized for each kilogram of extra body fat. Thus, a 10-kg increase in body fat leads to the daily synthesis of as much cholesterol as contained in the yolk of one egg. The increased cholesterol is in turn excreted in the bile, where high cholesterol concentrations relative to bile acids and phospholipids increase the likelihood of precipitation of cholesterol gallstones in the gallbladder (see Chapter 65). Additional factors, however, such as nidation conditions, also are involved in determining whether gallstones form.60 During weight loss, the likelihood of gallstone formation increases because the flux of cholesterol mobilized from fat is increased through the biliary system. Diets with moderate levels of fat that trigger gallbladder contraction and thus empty its cholesterol content may reduce this risk. Similarly, the use of bile acids, such as ursodeoxycholic acid, may be advisable if the risk of gallstone formation is thought to be increased.
Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is the term given to describe a constellation of liver abnormalities associated with overweight, including hepatomegaly, elevated liver biochemical test results, and abnormal liver histology, including steatosis, steatohepatitis, fibrosis, and cirrhosis (see Chapter 85).61 NAFLD may reflect increased very low-density lipoprotein (VLDL) production associated with hyperinsulinemia. A study using a cross-sectional analysis of liver biopsies has suggested that in overweight patients, the prevalences of steatosis, steatohepatitis, and cirrhosis are approximately 75%, 20%, and 2% respectively.62 Data from the Homeostasis Assessment Model (HOMA), a means used to develop mathematical models to describe glucose regulation, have shown that the more marked the insulin resistance, the higher the prevalence of severe steatosis.63 Using ultrasound for diagnosing increased liver fat, Hamaguchi and colleagues64 found that in a Japanese population there was a 10% incidence of new cases of NAFLD after a mean follow-up of 414 days, and that this was predicted by the presence of the metabolic syndrome. If increased fat in the liver is suspected, an ultrasound of the liver can provide a quantitative estimate that is much better than serum liver biochemical test results.
Gastroesophageal Reflux Disease
Overweight also may be a contributing factor in gastroesophageal reflux disease (GERD) (see Chapter 43). A total of nine studies examined the association of GERD with BMI,65 six of which showed a statistically significant association. Erosive esophagitis and esophageal adenocarcinoma also were more common in obesity. The odds ratio for GERD was 1.43 in the overweight group (BMI, 25 to 29.9 kg/m2) compared with the normal-weight group and rose to 1.94 when the BMI was higher than 30 kg/m2.66
Cancer
Certain forms of cancer are significantly increased in obesity.67–69 Obese men face increased risk for neoplasms of the colon, rectum, and prostate, whereas in women, cancers of the reproductive system and gallbladder are more common than in nonobese women. One explanation for the increased risk of endometrial cancer in overweight women is the increased production of estrogens by stromal cells in adipose tissue. This increased production is related to the degree of excess body fat and accounts for a major source of estrogen production in postmenopausal women. Breast cancer is not only related to total body fat, but also may have a more important relationship to central body fat,70 which may help explain why breast cancer risk is increased at age 75 in women in the highest versus the lowest quartile of BMI.71 Increased visceral fat as measured by CT shows an important relationship to the risk of breast cancer.
The Nurses Health Study has added significant insight to the relationship of body weight and breast cancer. Women who gained 25 kg or more after age 18 were at increased risk of breast cancer (relative risk [RR], 1.45; P < 0.001), and women who gained 10 kg or more after menopause were at increased risk for breast cancer compared with women whose weight remained stable. Women who achieved and maintained a 10-kg or more weight loss and who did not use postmenopausal hormones were at lower risk than those who maintained a stable weight.72 Finally, a pooling project with data from 13 cohort studies found that the relative risk of renal cell carcinoma was increased to 2.10 in those with a BMI > 30 kg/m2 compared with those with a BMI < 23 kg/m2.73
Endocrine Effects
A variety of endocrine changes are associated with obesity, including the polycystic ovary syndrome (PCOS), which is characterized by hirsutism, oligomenorrhea, and marked insulin resistance, hyperactivity of the adrenal glands (Cushing’s syndrome), and reduced fertility in men and women. In the Nurses Health Study, as BMI increased, the relative risk of infertility rose. Compared with the reference group, which had a BMI of 20 to 21.9 kg/m2, the relative risk of infertility was 1.7 for a BMI of 26 to 27.9 kg/m2 and 2.7 for a BMI above 30 kg/m2.74
Obesity influences the outcome of pregnancy. Increasing prepregnancy body weight was associated with a significant and weight-related increase in the likelihood of cesarean delivery. Infant pre-term birth weight was higher in smaller women. Heavier women had heavier babies, but no increased risk of low birth weight infants. Low birth weight infants were less likely in heavier women and also in those who gain more weight during their pregnancy.75 Weight gain of more than18.6 kg (41 pounds) also increases the risk of cesarean delivery. The risk of postpartum urinary tract infection also appears to be increased in overweight women, based on an observational study of 60,167 women.76
In a large retrospective study from Scotland, nulliparous women, compared with multiparous women, had increased elective preterm delivery, neonatal death, and infant weights less than 1000 g, and these effects were greatest in women with a BMI > 35 kg/m2.77
Pneumonia
Community-acquired pneumonia may be an additional risk related to being obese. In the Health Professionals Follow-up Study and the Nurses Health Study II, the risk of pneumonia increased as BMI increased.78 Significant weight gain in women after age 18 years also increased the risk of pneumonia.