Nutritional Intervention in Progressive Chronic Kidney Disease
Denis Fouque
Fitsum Guebre-Egziabher
Lipids and carbohydrates are completely processed or stored in the body so protein and its nitrogen derivatives are the most important food-derived compounds handled by the kidney. At equilibrium, every gram of nitrogen from the diet is absorbed and rapidly eliminated into the urine. This property allows easy monitoring of protein intake by measuring excreted nitrogen (or urea) in urine. In healthy adults, increasing protein intake is associated with a concomitant increase in nitrogen output, whereas a diet with inadequate protein is associated with a markedly reduced urinary urea nitrogen output. This adaptive ability is limited because the body has obligatory daily nitrogen losses that are not influenced by protein intake, for example, nitrogen losses in feces, perspiration, hair, and nails. In patients with kidney disease, this loss has been estimated to be approximately 0.031 mg nitrogen per kg of body weight.
Protein requirements are estimated by standard methods, such as nitrogen balance or labeled amino acid turnover. These methods can characterize body nitrogen metabolism and the optimal level of protein intake for healthy adults and patients with chronic kidney disease (CKD). From these data, safe diets are devised for patients with varying levels of kidney disease.
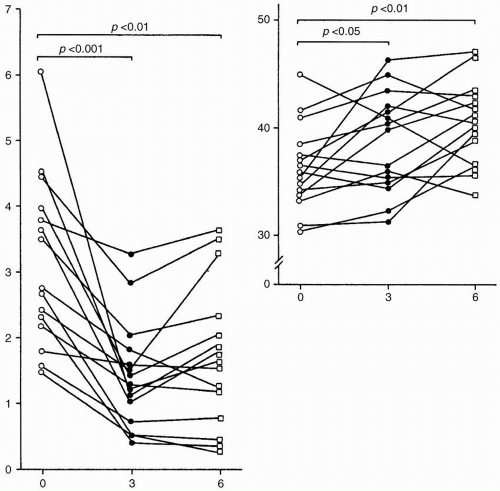
Protein intake influences the degree of proteinuria (Fig. 9-1). Because proteinuria has been identified as one of the most important and independent risk factors for CKD progression, every attempt to lower proteinuria to minimal levels seems worthwhile. We will address the potential influence of protein intake on kidney function, the optimal dietary protein for patients with CKD who have various levels of kidney function, the ways to monitor such diets, the potential side effects, and the results of large clinical trials and meta-analyses of low-protein dietary interventions on the course of CKD.
PROTEIN METABOLISM AND KIDNEY DISEASE
Overnutrition is associated with altered renal hemodynamics, particularly when there is a high level of protein or amino acid intake. Whether lipids or carbohydrates directly affect kidney function or disease is unclear, but there is ample evidence that a high protein intake will acutely increase glomerular filtration rate (GFR) and urinary albumin excretion. With long-term dietary restriction, protein intake also influences the degree of glomerulosclerosis. By contrast, reducing the protein load may stop or even induce remission in the progression of experimental renal scarring. Several mechanisms or compounds have been proposed to explain these alterations, including glucagon, insulin, insulin-like
growth factor-1, angiotension II, prostaglandins, and kinins. Sodium retention may also be associated with protein-induced hyperfiltration via activity of the proximal, sodium-amino acid cotransporter. Protein restriction ablates hemodynamic changes related to 5/6 nephrectomy because it reduces glomerular pressure and flow.
growth factor-1, angiotension II, prostaglandins, and kinins. Sodium retention may also be associated with protein-induced hyperfiltration via activity of the proximal, sodium-amino acid cotransporter. Protein restriction ablates hemodynamic changes related to 5/6 nephrectomy because it reduces glomerular pressure and flow.
Glomerular protein trafficking induces hypermetabolism and oxidant stress, while a low-protein diet (LPD) reduces oxygen consumption and malondialdehyde production. The nature of protein absorbed may also influence the renal response because the GFR of vegetarians is reportedly lower than that of omnivores. When rats with CKD were fed casein or soy proteins, proteinuria and histologic renal damage were always more severe compared with results in rats fed protein of animal origin.
Levels of growth factors and profibrotic agents, such as transforming growth factor (TGF)-beta, fibronectin, and plasminogen activator inhibitor-1 (PAI-1) are modulated by an LPD, resulting in reduced proteinuria and kidney injury. Finally, albuminuria per se possesses pathogenic effects promoting tubulointerstitial scar and apoptosis, and LPD can independently decrease glomerular
capillary pressure and albuminuria, adding a protective antifibrotic and antiapoptotic effect.
capillary pressure and albuminuria, adding a protective antifibrotic and antiapoptotic effect.
OPTIMAL LEVEL OF DIETARY PROTEIN AND ENERGY INTAKE
A Western diet contains approximately 1.4 g protein/kg b.w./day; women have a daily protein intake that is 35% to 50% lower than men because of their lower body weight, and aging also affects protein intake; it is spontaneously reduced 15% by the age of 70 years. The mean optimal protein intake, according to the Food and Agriculture Organization (FAO) in 1985, is 0.50 g protein/kg b.w./day. To ensure that 97.5% of individuals maintain nitrogen balance, two standard deviations of the mean are added for an optimal daily intake to 0.75 g protein/kg b.w./day. To correct for vegetable protein (less absorption by a factor of 10%), the optimal protein intake was raised to 0.8 g protein/kg b.w./day. More recent research using sophisticated measurements of protein turnover has confirmed these values. There is a Gaussian distribution of protein requirements, however, explaining why some patients do well with protein intakes below the average recommended values. Finally, the protein requirements have been estimated in healthy adults or in patients with kidney disease who were receiving a controlled energy intake of at least 35 kcal/kg b.w./day, and the diet may not be applicable to healthy adults or patients with CKD with a reduced energy intake.
Interestingly, the basal metabolic rate and energy requirements in patients with CKD who are not on maintenance dialysis do not differ from those in healthy adults, and nitrogen balance is obtained when a protein intake is 0.6 g/kg b.w./day. In 2000, the Kidney Disease Outcomes Quality Initiatives (K/DOQI) of the National Kidney Foundation recommended that protein and energy intake should be 0.6 g protein/kg b.w./day (Guideline 24) and energy intake, 35 kcal/kg b.w./day for patients <60 years with CKD and 30 to 35 kcal/kg b.w./day for patients >60 with CKD (Guideline 25). These recommendations change when maintanence dialysis treatment is initiated. However, there are spontaneous reductions in energy and protein intake when kidney function decreases leading to intake values as low as 21 kcal and 0.85 g protein/kg b.w./day in patients with stage 3 CKD (estimated GFR <30 mL per minute). Notably, for an equal amount of protein, a diet with 70% of protein from vegetable sources enables a greater amount of calories to be consumed than a diet consisting of 70% of its protein from animal sources. Because energy intake may decrease when kidney function falls, a defect in protein metabolism may occur with an inadequate dietary energy intake. Obviously, patients with CKD will need instruction in methods for achieving an optimal, moderately low protein intake and adequate energy intake.
METABOLIC CONSEQUENCES OF REDUCED PROTEIN INTAKE IN CHRONIC KIDNEY DISEASE
Nature of Protein Restricted Diets
Healthy adults or patients with CKD have been shown to adapt to a dietary protein intake as low as 0.3 g protein/kg b.w./day, if
energy and essential amino acid (EAA) requirements are met. To avoid nutritional deficits, supplements of EAA or ketoacids (KA) of amino acids have been suggested; the ketoacids are synthesized into the corresponding EAAs. Without these supplements, intakes as low as 0.6 g protein/kg b.w./day can be safely used if at least 50% of the protein is of high biologic value (e.g., principally from animal sources) and energy intake meets the recommended goal (i.e., 35 kcal/kg b.w./day for patients younger than 65 years and 30 to 35 kcal/kg b.w./day for those >65 years). If lower levels of protein intake are prescribed, supplements (EAA or KA) should be considered to avoid inducing an EAA deficit. The potential benefits are that low dietary protein intake can slow the progression of CKD and alleviate uremic symptoms and postpone the start of maintenance dialysis or prolong survival when dialysis opportunities are limited.
energy and essential amino acid (EAA) requirements are met. To avoid nutritional deficits, supplements of EAA or ketoacids (KA) of amino acids have been suggested; the ketoacids are synthesized into the corresponding EAAs. Without these supplements, intakes as low as 0.6 g protein/kg b.w./day can be safely used if at least 50% of the protein is of high biologic value (e.g., principally from animal sources) and energy intake meets the recommended goal (i.e., 35 kcal/kg b.w./day for patients younger than 65 years and 30 to 35 kcal/kg b.w./day for those >65 years). If lower levels of protein intake are prescribed, supplements (EAA or KA) should be considered to avoid inducing an EAA deficit. The potential benefits are that low dietary protein intake can slow the progression of CKD and alleviate uremic symptoms and postpone the start of maintenance dialysis or prolong survival when dialysis opportunities are limited.
Adaptation to Protein Metabolism
An adaptive response (i.e., a decrease in whole body leucine flux and oxidation) occurs in patients with stage 3 to 4 CKD who had a 40% reduction in their dietary protein intake (i.e., from 1.1 to 0.7 g/kg/day) during a period of 3 months; body composition is maintained. This adaptive response was also observed with a more restricted protein intake (0.35 g/kg/day supplemented with KA) treated over an extended period of 16 months. The adaptation has also been observed in patients with CKD with nephrotic syndrome when their protein intake was reduced from 1.85 to 1.0 g protein/kg/day. The LPD was even more beneficial in nephrotic patients with CKD who were tested while their intake was reduced from 1.20 to 0.66 g protein/kg/day; amino acid metabolism improved, and there was strong reduction in proteinuria leading to increased serum albumin concentrations. Thus, with adequate energy supply, patients with CKD correctly adapt their protein metabolism.
Glucose Metabolism
Insulin resistance is common during the course of CKD, and glycemic control can improve with attention to nutrition. After 3 months of an LPD, insulin sensitivity improved and there was reduced fasting serum insulin or daily insulin needs and blood glucose values and endogenous glucose production. The mechanisms for these benefits are unclear, but the results are encouraging.
Control of Chronic Kidney Disease—Mineral and Bone Disorders
Because proteins of animal origin are strongly associated with phosphates (1 g protein approximately contains 13 mg of phosphorus), limiting dietary protein intake reduces the phosphate intake and hence the amount that must be excreted. With calcium salts of KA as a supplement, there also is extra calcium. Both low phosphate intake and calcium supplements reduce serum parathormone (PTH) levels and improve renal osteodystrophy, and this has been shown during 12 months of an LPD supplemented with KA. Given the recent reports regarding the adverse effects of excessive calcium intake in patients with advanced
CKD, use of calcium containing KA supplements must be used with caution.
CKD, use of calcium containing KA supplements must be used with caution.
Improvement in Lipid Profile
The reduced protein intake of animal origin (e.g., meat and dairy products) generally entails a decrease in saturated lipids, leading to an improvement in the overall serum lipid profile. For example, reducing protein intake for 3 months from 1.1 to 0.7 g/kg/day resulted in an increase in serum lipoprotein AI and in the Apo-AI/Apo-B ratio, changes considered to reduce the cardiovascular risk in general population. Likewise, a 6-month LPD regimen improved decreased red cell malondialdehyde and increasing polyunsaturated fatty acids, particularly C22:4 and C22:5, thereby limiting oxidative stress.
Reduction in Proteinuria and Amelioration of Kidney Injury
Lowering dietary protein intake induces a decrease in proteinuria (see Fig. 9-1). Because proteinuria is now identified as an independent risk factor for progression of CKD, every attempt to reverse proteinuria is worthwhile. Whether reducing protein intake will protect the kidney from progressive injury in humans is less clear. First, experimental studies used diets with extreme differences in protein intake. Obviously, the clinical use of these diets does not permit this much variation. Second, protein in food is associated with other factors such as phosphorus, sodium, energy, and water that could affect kidney function. Third, a single intervention can be studied experimentally, but in clinical practice, patients generally receive several nephroprotective interventions that may mask the true effect of the diet as a single intervention. Besides angiotensin-converting enzyme (ACE) inhibitors, AT1 receptor blockers, or both, an LPD might confer additional protection against progression of kidney disease and may potentuially postpone end-stage renal disease (ESRD).
Hypocaloric Risk
Without adequate counseling, too few calories may be contained in the protein-restricted diet. With adequate supervision, however, energy intakes are usually greater than 30 kcal/kg/day, and body composition is unchanged during long-term LPD therapy. However, clinical trials have led to the conclusion that even at fairly low energy intake, long-term nutritional status, as measured by dual-energy x-ray absorptiometry (DEXA) or anthropometry, does not reveal significant changes. Furthermore, patients treated with LPD for years had a survival rate during maintenance dialysis similar to that achieved by other therapies.
MONITORING NUTRITIONAL INTAKE
Protein intake can be estimated from two sources: (i) intake from dietary reports; and (ii) output from urinary urea excretion in patients without ESRD, or protein nitrogen appearance (PNA), formerly called protein catabolic rate (PCR). Two formulas are routinely used to assess nitrogen (N) and thus protein intake in CKD:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree