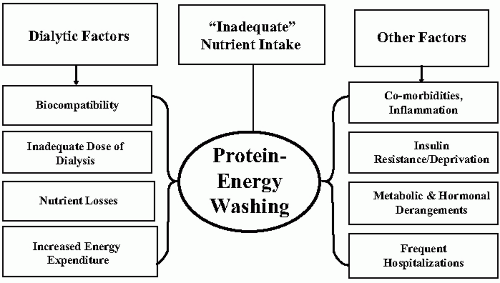
Among the risk factors that affect patients with end-stage renal disease (ESRD) and especially those receiving maintenance hemodialysis (MHD), metabolic and nutritional derangements designated as protein-energy wasting (PEW) of chronic kidney disease (CKD) plays an important role. PEW is associated with major adverse clinical outcomes such as increased rates of hospitalization and death in patients undergoing MHD. For these reasons, the prevention and treatment options of PEW are both critical and complex in MHD patients. Because of the large number of factors affecting nutritional and metabolic status of patients undergoing MHD (
Fig. 11-1), prevention and treatment of PEW requires a comprehensive combination of strategies to prevent protein and energy depletion and to institute therapies that will avoid further losses.
DIAGNOSIS OF PROTEIN-ENERGY WASTING IN PATIENTS UNDERGOING MAINTENANCE HEMODIALYSIS
A summary of nutritional parameters for detecting PEW in patients undergoing conventional hemodialysis (CHD) and their applicability for guiding nutritional therapies is provided in
Table 11-1. Recently, the International Society of Renal Nutrition and Metabolism organized an expert panel to re-examine the terms and criteria used for the diagnosis of PEW; the recommendations for the four main, established categories for the diagnosis of PEW are in Chapter 14 (
Table 14-1).
Assessment of Visceral Protein Stores
Serum albumin has been used most widely as a nutritional marker in patients receiving MHD. This is primarily because of its easy availability and strong association with hospitalization and death risk. Unfortunately, there are many nonnutritional factors that influence serum albumin concentrations such as decreased synthesis from liver diseases, increased transcapillary losses, and increased losses from the gastrointestinal tract and kidneys and from tissue injuries such as wounds, burns, and peritonitis. Serum albumin has also been shown to decrease with volume overload, a highly prevalent condition in patients undergoing MHD. Serum albumin (a “negative acute-phase reactant”) is also affected by conditions such as inflammation, infection, and trauma, which decrease albumin synthesis, leading to prompt decreases in serum albumin concentration. Consequently, the decrease in serum albumin can reflect the degree of illness and inflammation, rather than nutritional status. Because a low serum albumin is highly predictive of poor clinical outcomes at all stages of CKD, it is still considered a reliable index that there can be abnormalities in a patient’s nutritional status.
Serum transferrin is another potential nutritional biomarker, but its level is influenced by changes in iron stores, the presence of inflammation, and changes in extracellular volume. Consequently, it is not a good indicator of nutritional status. Although very responsive to dietary nutrient intake, serum prealbumin levels may also rise because of decreased kidney function. In addition, prealbumin is a negative acute-phase reactant, so its utility in monitoring nutritional status in MHD patients is questionable. Since serum albumin is also a negative acute phase reactant like prealbumin, it is reasonable to measure the C-reactive protein (CRP), an acute-phase reactant and an index of inflammation when serum levels of albumin or prealbumin are low.
Assessment of Body Composition
The assessment of body composition and somatic protein stores is based on the measurement of different body compartments (water, fat, bone, muscle, and visceral organs). The fat-free mass (mainly composed of muscle) is the major somatic protein mass. Generally, somatic protein stores are preserved, but when there are catabolic illnesses, depletion of fat-free mass will occur. We use body composition techniques to diagnose protein depletion and to monitor efficacy of nutritional therapies. Simple anthropometric measures can provide a guide over long-term treatment, and bioelectrical impedance analysis (BIA) and dual-energy x-ray absorptiometry (DEXA) can be used for research purposes as long as it is recognized that both of these techniques are greatly influenced by changes in body water, a complication that is common in MHD patients.
Composite Indices of Nutritional Status
The diagnostic measures based on changes in body composition and dietary intake assessment have been accompanied by a subjective assessment of overall nutritional status, especially when large numbers of patients are being evaluated. The most commonly
used composite indices include subjective global assessment (SGA) or its modified or expanded versions such as the composite nutritional index (CNI) and the malnutrition-inflammation score (MIS). These measures can only provide a general assessment because they evaluate nutritional status from a broader perspective, including medical history, symptoms, and physical parameters.
SGA was originally used to evaluate outcomes in surgical patients with gastrointestinal disease. The evaluation included a history of weight changes, nutritional intake and gastrointestinal symptoms, nutrition-related functional impairment plus a physical examination to assess subcutaneous fat, muscle stores, and the presence or absence of edema. The surgical patients were divided into three categories: (i) well-nourished; (ii) mild to moderately malnourished; or (iii) severely malnourished. In the surgical patients, the SGA was found to correlate with other measures of nutritional status and clinical outcome. The use of the SGA was expanded to include patients undergoing MHD, but the SGA does not reliably detect sarcopenia (based on total body nitrogen), and even though it has some utility in discriminating between best- versus worst-nourished patients, the SGA does not separate patients undergoing MHD with mild or moderate PEW. Despite these shortcomings, the National Kidney Foundation Disease Outcomes Quality Initiative (NKF K/DOQI) guidelines included a recommendation that the modified SGA should be performed every 6 months in patients receiving MHD. However, its subjective nature, the absence of measures of visceral protein stores, and its relative insensitivity to small changes in nutritional status greatly limit the usefulness of the SGA. We believe that if used, the SGA should be accompanied by parameters that include body weight and weight-for-height, skinfold measures, and serum albumin concentration plus other estimates of protein stores.
The Malnutrition Inflammation Score (MIS) incorporates components of the SGA and includes components related to nutritional status (body mass index) and inflammation (serum albumin concentration and total iron binding capacity) plus indices that are not directly related to nutritional status, such as comorbidities and functional status. We recommend that the MIS should be used in conjunction with other methods as proposed for SGA.
The critical point is that the nutritional status of patients undergoing MHD should be monitored regularly to detect nutritional disturbances early. In addition, regular monitoring is needed to evaluate the response of nutritional interventions and to motivate and improve patient’s compliance to the dietary therapy. Overall, we recommend routine follow-up measurements that include body weight and nPNA plus values of serum albumin, prealbumin, and cholesterol every 3 months in clinically stable patients. Some investigators include anthropometric measurements, dietary interviews, and SGA every 6 months for those patients who are at risk of developing PEW or with established PEW, but the utility of this strategy has not been evaluated carefully.
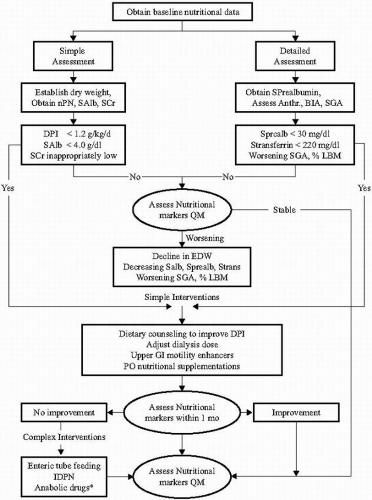
Figure 11-2 depicts a proposed algorithm for the assessment and management of nutritional status in patients receiving MHD.
EPIDEMIOLOGY OF PROTEIN-ENERGY WASTING IN PATIENTS UNDERGOING MAINTENANCE HEMODIALYSIS
Virtually every study evaluating the nutritional status of MHD patients reports some degree of abnormalities in the nutritional status. Unfortunately, many different diagnostic tools were used in the separate studies, so the actual prevalence of PEW in MHD patients varies widely, ranging from 20% to 60%. Although there is evidence that nutritional parameters improve within 3 to 6 months following initiation of hemodialysis, there also is evidence that PEW is present in up to 40% or more of the MHD population; the prevalence seems to increase with time of MHD treatment.
As noted above, there are serious shortcomings in the use of serum albumin as a principal measure of nutritional status. Unfortunately, serum albumin has been the major basis for epidemiologic reports on nutrition in patients undergoing MHD. For example, in the baseline phase of the Hemodialysis (HEMO) Study, 29% of the patients had albumin levels below 3.5 g/dL. Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) suggest there is a lower prevalence of hypoalbuminemia in countries other than United States, with the lowest mean value of serum albumin occurring in the United Kingdom for Europe and the U.S. value being significantly lower compared with European countries (3.60 versus 3.72 g/dL [36 versus 37 g/L]). Japan also had significantly higher albumin values compared with U.S. values even when adjusted for patient age, sex, and the timing of the measurement (i.e., during the week or over the weekend). In DOPPS II, 20.5% of U.S. patients had a serum albumin level less than 3.5 g/dL (35 g/L). Based on the serum albumin and the SGA, it was concluded that the prevalence of moderate malnutrition ranged from 7.6% (United States) to 18% (France) and for severe malnutrition, from 2.3% (Italy) to 11% (United States). Because these conclusions are made based on inaccurate assessments of nutritional status, their usefulness in understanding what occurs in patients undergoing MHD is very limited. On the other hand, the relevance of these measurements is that practically every marker has been associated with hospitalization and death risk. Recent epidemiologic data also indicate there is an improvement in survival when these markers are corrected.
Etiology of Protein-Energy Wasting in Patients Receiving Maintenance Hemodialysis
The mechanisms leading to PEW in advanced kidney disease are still being elucidated; they can not be attributed to any single factor in patients receiving MHD (
Fig. 11-1). Still, it appears that the common pathway for all the metabolic derangements is related to exaggerated protein degradation relative to decreased protein synthesis. There are no agreed upon mechanisms relating dietary protein and energy intake and the development of nutritional and metabolic abnormalities in CHD, but there is an epidemiological association between inadequate nutrient intake and the abnormalities associated with PEW.
Dietary Nutrient Intake, Hemodialysis, and Development of Protein-Energy Wasting in Patients Receiving Maintenance Hemodialysis
The observation that patients with CKD decrease their protein and energy intake as they progressively lose kidney function has led some to conclude that uremia per se causes protein catabolism stimulated by decreased nutrient intake. This conclusion has been challenged because even in patients with advanced CKD, balance studies show that there is a concomitant decrease in both protein synthesis and degradation that is at least uncomplicated by acidosis. The dual change in protein synthesis and degradation results in a net nitrogen balance that is not different from matched healthy controls. Conversely, accelerated protein degradation stimulated by acute illnesses or stress conditions is not suppressed, and there is no adequate compensatory increase in protein synthesis partly caused by insufficient dietary nutrient intake. For example, hospitalized patients undergoing MHD can be provided limited protein and energy and may not be able to adjust rates of protein turnover leading to loss of cellular protein stores.
An additional stimulus for protein losses is the dialytic treatment per se. Recent measurements of protein synthesis and degradation unequivocally demonstrate the catabolic effects of hemodialysis. Both whole-body and skeletal muscle protein homeostasis are disrupted, and there is a consistent finding of decreased protein synthesis at the whole-body level and an additional increase in whole-body protein breakdown. There also is evidence for a significant increase in net skeletal muscle protein breakdown. Notably, these undesirable effects persist for at least 2 hours following the completion of hemodialysis.