Fig. 4.1
The kinetics of TRAIL expression on liver NK cells after hepatectomy. (a) The numbers presented are means ± SEM of the TRAIL-positive cell population in NK cell subsets. *p < 0.01 for day 3, day 7, and day 14 compared to the control. (b) The numbers indicate the mean fluorescence intensity of cells that stained positive for TRAIL. *p < 0.01 for day 1 and day 3 compared to the control
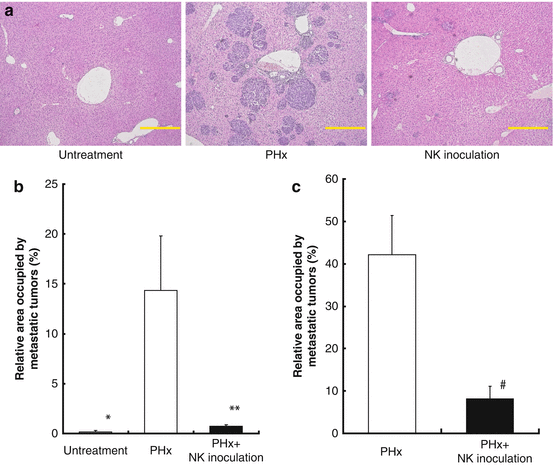
Fig. 4.2
The adoptive transfer of activated liver NK cells inhibited the growth of liver metastasis induced by a portal venous injection of hepatoma cells in extensively hepatectomized mice. (a) Representative histopathological findings of the liver specimen. Specimens from the untreated (left), partially hepatectomized (PHx; middle), and NK cell-receiving groups that were inoculated after partial hepatectomy (right). The adoptive transfer of activated B6 liver NK cells inhibited the growth of liver metastasis induced by a portal venous injection of hepatoma cells (Hepa 1–6; 5 × 105 cells) in extensively hepatectomized B6 mice (b) and B6CF1 mice (c)
4.3.2 Liver NK Cells in Human
Differences between liver and peripheral blood NK cells have not been extensively investigated in human because of the limited availability of appropriate human samples. We have recently determined the phenotypic and functional properties of NK cells extracted from donor and recipient liver perfusate in clinical living donor liver transplantation [10]. Donor liver NK cells exhibited the most vigorous cytotoxicity against a hepatocellular carcinoma cell line after in vitro IL-2 stimulation, compared to the activities of donor and recipient peripheral blood NK cells and recipient liver NK cells (Fig. 4.3). IL-2 stimulation increased TRAIL expression, which has been shown to be critical for NK cell-mediated antitumor cell killing without affecting normal cells, on liver NK cells (Fig. 4.4) [31, 32]. In addition, we have confirmed that death-inducing TRAIL receptors, TRAIL-R1/death receptor (DR) 4, and TRAIL-R2/DR5, which contain cytoplasmic death domains and signal apoptosis, are expressed in HCC [33, 34]. These findings suggest a novel concept to prevent recurrence of HCC after liver transplantation, the adoptive transfer of IL-2-stimulated NK cells extracted from donor liver graft into recipients.
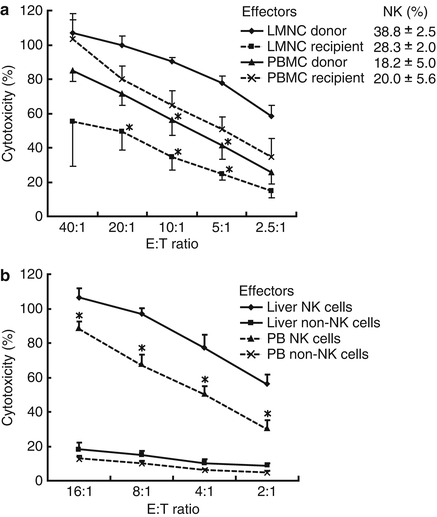
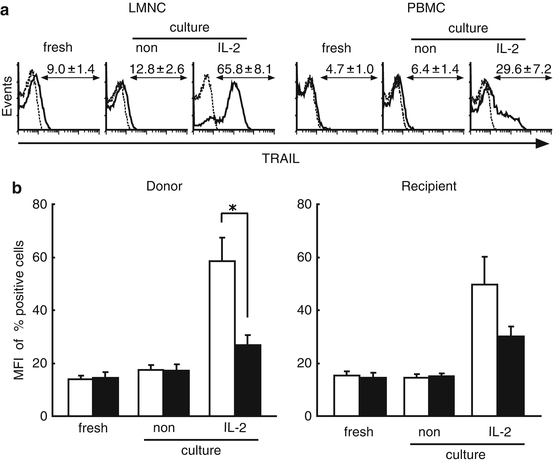

Fig. 4.3
IL-2-stimulated donor liver NK cells show vigorous cytotoxicity against HepG2 cells. NK cytotoxic activities of indicated effectors against indicated target cells were analyzed by 51Cr release assay. (a) NK cytotoxic activities of IL-2-stimulated liver mononuclear cell (LMNC) and peripheral blood mononuclear cell (PBMC) populations that were cultivated for 4 days in the presence of IL-2 before the cytotoxic assay. Percentages of CD3−CD56+ NK cells in LMNC or PBMC fractions obtained from 4 adult healthy donors and 4 corresponding recipients with cirrhosis are shown in the upper right corner (means ± SEM). (b) NK cytotoxic activities of NK and non-NK cells isolated from IL-2-stimulated donor LMNC and PBMC populations against HepG2 target cells

Fig. 4.4
Liver NK cells inductively express remarkable levels of TRAIL, but peripheral blood NK cells do not. Freshly isolated LMNC and PBMC fractions obtained from healthy donors and corresponding recipients cultivated with of without IL-2 were stained with CD3 and CD56 monoclonal antibodies (MAb) together with TRAIL MAb. (a) Representative histograms show the log fluorescence intensities obtained upon staining for TRAIL after gating on the CD3−CD56+ NK cells subsets of healthy donors. Dotted lines represent negative control staining with isotype-matched MAbs. The numbers indicate the percentages of cells in each group that were positive for TRAIL expression. (b) The numbers indicate the mean fluorescence intensity (MFI) of cells in each group that stained positively for TRAIL on freshly isolated NK cells cultivated with/without IL-2 (LMNC; open column, PBMC; closed column). The data are presented as means ± SEM. Statistical analyses were performed using ANOVA (*p < 0.05)
4.4 Clinical-Scale Isolation of Liver NK Cells from Donor Liver Graft
In order to apply this NK cell immunotherapy to cases of deceased donor liver transplantation, we demonstrated for the first time the phenotypical and functional properties of liver NK cells that were extracted from a deceased donor liver graft perfusate under current good manufacturing practice conditions (cGMP) [35]. First, a large number of NK and NKT cells were verified among liver mononuclear cells, with both subsets possessing characteristics different from those of peripheral blood mononuclear cells. Second, in vitro stimulation with IL-2 induced liver NK cells to strongly upregulate activation markers, cytotoxicity, and cytokine production while maintaining the expression of inhibitory receptors. These results were compatible with those for a living donor liver graft perfusate [10]. Finally, we confirmed that the final product met the lot release criteria and contained a low T-cell number, thereby reducing the possibility of graft versus host disease (GVHD) in a recipient.
4.4.1 Protocol of NK Isolation
At the time of donor operation, the graft liver was placed in a bag and perfused through the portal vein with 2 L of University of Wisconsin cold storage solution (UW solution) and crystalloids. This perfusate was collected from the hepatic vein and retrieved in our cGMP cell processing facility. Since the UW solution has a high viscosity [36], the perfusate was centrifuged at 2800 × g for 30 min at 4 °C to ensure adequate centrifugation. The cell pellet was then subjected to a Ficoll-Hypaque density gradient centrifugation to separate mononuclear cells. Liver mononuclear cells were cultured with human recombinant IL-2 in complete medium at 37 °C in atmosphere supplemented with 5 % CO2 for 3–5 days. Anti-CD3 monoclonal antibody was added to the culture medium in order to deplete the CD3+ fraction 1 day prior to cell harvesting. On the day of infusion, the cells were washed twice with 0.9 % sodium chloride and resuspended in 5 % human serum albumin for injection. Testing for lot release included cell counts, viability assessment, Gram stain, and endotoxin analysis (Fig. 4.5).
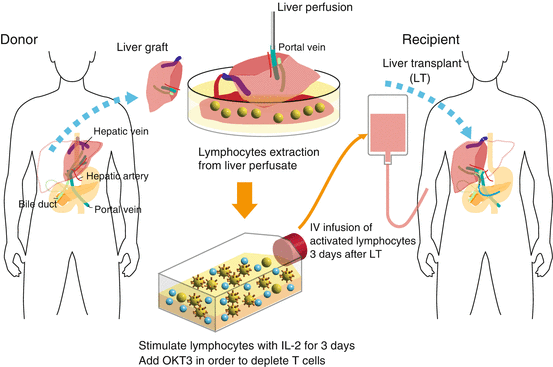

Fig. 4.5
Schematic outline of adoptive immunotherapy with lymphocytes extracted from liver allograft perfusate
4.5 Clinical Trials for Living and Deceased Donor Liver Transplantation
On the basis of these results, we started a clinical trial of adjuvant immunotherapy with activated donor liver NK cells for preventing the recurrence of HCC after living donor liver transplantation in 2006. The lymphocytes in the peripheral blood of liver transplant recipients who received immunotherapy in the early postoperative period showed significantly enhanced cytotoxicity against an HCC cell line (HepG2), compared with those in the peripheral blood of liver transplant recipients who did not receive the therapy in the same period (Fig. 4.6a). Correspondingly, the proportions of TRAIL+ NK cells in the peripheral blood of liver transplant recipient who received immunotherapy also significantly increased (Fig. 4.6b). Although the long-term benefits of this approach with regard to HCC recurrence after liver transplantation remain to be elucidated, there were no severe adverse events including GVHD, thus far. With the collaboration of Professors A. Tzakis and S. Nishida at the University of Miami, FL, we successfully performed a phase I study of adoptive immunotherapy using liver NK cells for deceased donor liver transplant recipients with HCC (ClinicalTrials.gov identifier: NCT01147380) beginning in 2009. Both clinical studies are under evaluation and these results will be published soon.
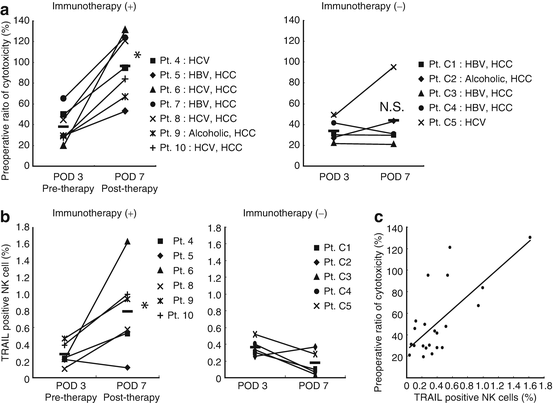

Fig. 4.6
Adoptive immunotherapy with activated donor liver NK cells promoted the cytotoxic activity and TRAIL expression of NK cells in liver transplant recipients. (a) The NK-mediated cytotoxic activities of the indicated effectors against their target cells were analyzed by 51Cr-release assay. The dot plot represents the NK cytotoxic activities of freshly isolated peripheral blood lymphocytes obtained from recipients who received immunotherapy (+) and did not receive immunotherapy (−) against HepG2 target cells (effector/target ratio, 40:1) 3 and 7 days after liver transplantation. NK cytotoxic activities are presented as a proportion (percentage) of the preoperative cytotoxicity in each patient. Horizontal lines indicate the mean.*p < 0.05 for day 7 compared to day 3. (b) The frequency of TRAIL + NK cells increased remarkably in the peripheral blood of liver transplant recipients who received immunotherapy. *p = 0.013 for the immunotherapy group compared to the untreated group on postoperative day 7. (c) Correlation between TRAIL+NK cell ratio and NK cytolytic activity after liver transplantation (Spearman rank-order correlation coefficient r = 0.54, p = 0.01)
References
1.
Horn M, Phebus C, Blatt J. Cancer chemotherapy after solid organ transplantation. Cancer. 1990;66(7):1468–71.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







