Nonneoplastic Diseases of the Appendix
Normal Embryonic Development
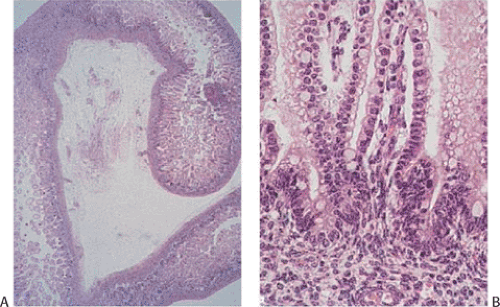
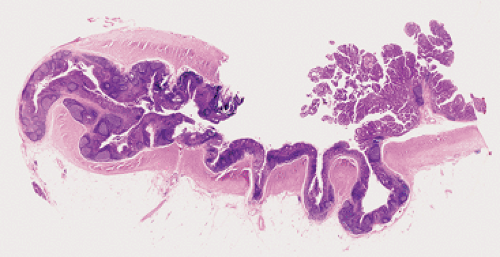
The cecum appears during the fifth fetal week, arising as a diverticulum from the distal primitive intestinal loop before it differentiates into the small and large intestine. The appendix develops from the cecum and matures in the second trimester (1). As the appendix lengthens, the junction between the appendix and the cecum becomes increasingly more distinct. Longitudinal folds and ridges form, producing a segmented appearance; villi form (Fig. 8.1) that eventually involute. The epithelium appears clear due to large amounts of intracytoplasmic glycogen. Endocrine cells develop in the subepithelial connective tissue around the ninth fetal week when the epithelial basement membrane is not fully formed and the muscularis mucosae has not yet developed.
Lymphoid stem cells migrate into the appendiceal mesenchyme. Mature lymphocytes appear when the fetus measures approximately 100 mm in length (2); lymphoid aggregates appear by the 17th week. The apical poles of incipient lymphoid follicles impinge on the surface epithelium by the time the fetus reaches 150 mm in length and lymphoid cells invade the epithelium. Germinal centers develop between the third and sixth postnatal weeks after the introduction of foreign protein (by eating) into the gut. Macrophages appear shortly after the lymphocytes (3). Primitive neural structures develop in the first trimester.
Normal Gross Anatomy
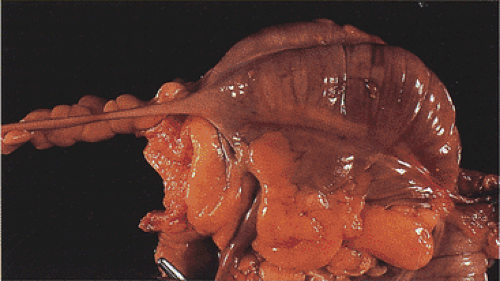
The appendix usually arises from the posteromedial cecal wall, 2.5 to 3 cm below the ileocecal valve at the convergence of the taeniae coli (Fig. 8.2). The adult appendix averages 7 cm in length; lengths up to 20 cm have been reported. The appendix is longer in adults than in children. Its external diameter ranges from 0.3 to 0.8 cm. The appendiceal lumen measures from 1 to 2 mm in diameter appearing round, oval, irregular, or slitlike. The distal tip obliterates in adults. The appendix is suspended from the mesoappendix, and attaches to the cecum in several ways (4). In 65% of adults, the appendix lies behind the cecum with its orifice opening into the cecum near the ileocecal valve. It may also lie to the side of the ascending colon, in front of, or behind, the ileum, lying on the psoas muscle or hanging over the pelvic brim (4). The appendix receives its blood supply from a branch of the posterior cecal artery; its venous drainage is to the portal system, explaining the coexistence of hepatic inflammation in the setting of appendicitis. The lymphatics first drain into the nodes of the mesoappendix and then to the right pericolic lymph nodes as well as to the ileocecal lymph nodes.
Histology of the Appendix
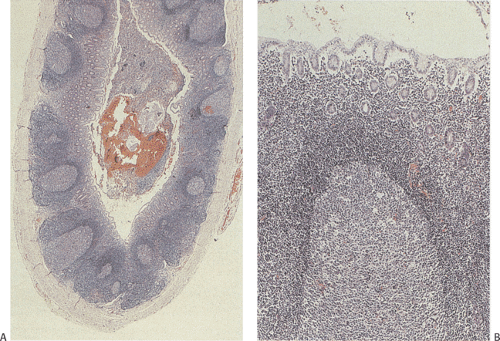
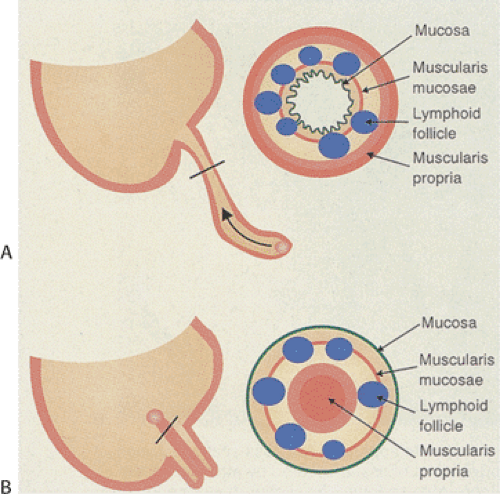
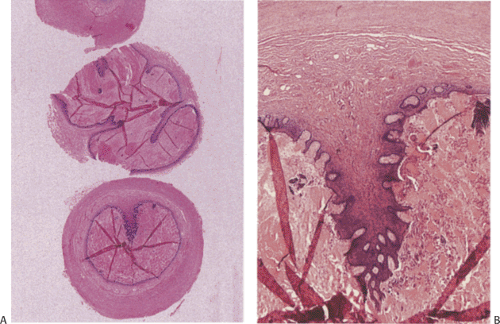
The appendiceal mucosa resembles that of the large intestine, except for the prominent circumferential arrangement of lymphoid follicles known as Peyer patches. Peyer patches (Fig 8.3) are most prominent in children, decreasing in size with age. They are markedly diminished or absent in the elderly. The appendiceal epithelium is modified over the dome of each lymphoid follicle to form M cells with a structure similar to that seen in the small intestine (see Chapter 6). Nonbranching crypts are lined by tall mucus-secreting goblet cells that extend from the luminal surface to the crypt bases. The crypts also contain endocrine cells, Paneth cells, and small numbers of intraepithelial lymphocytes. The muscularis mucosae is often absent; it is sometimes difficult to determine the mucosal–submucosal boundary.
The regularly arranged Peyer patches lie at the mucosal–submucosal junction (Fig. 8.3), and a well-defined lymphatic sinus surrounds both the lateral and basal parts of the follicle. These lymphatic sinuses empty into the submucosal-collecting lymphatics. Lymphocytes that proliferate in the gut-associated lymphoid tissues migrate into the surrounding lymphatic sinus or capillaries and enter the systemic circulation to be redistributed to other lymphoid tissues and organs. The endocrine cell population is discussed in Chapter 17.
Congenital Abnormalities of the Appendix
Congenital appendiceal anomalies are rare (5). They include appendiceal agenesis (6), hypoplasia, duplications, horseshoe shape (7), heterotopia, and diverticula. These
occur in the presence of either a normal cecum or with cecal dysgenesis.
occur in the presence of either a normal cecum or with cecal dysgenesis.
Appendiceal Agenesis
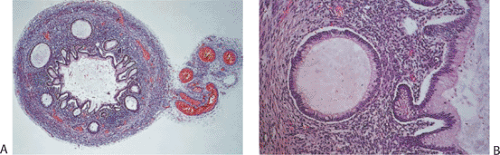
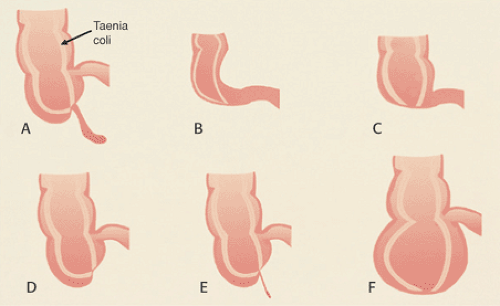
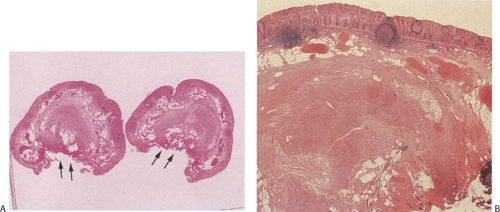
Appendiceal agenesis (or absence) differs from appendiceal hypoplasia in that in the latter condition, the appendix is present but underdeveloped with a simplified structure and with mucosal cysts (Fig. 8.4). There are five types of appendiceal agenesis (Fig. 8.5) (6). All result from failure of the primitive cecal diverticulum to differentiate into the appendix, except for the fourth type. The fourth type results from intrauterine atrophy of a previously well-formed appendix. Appendiceal agenesis sometimes accompanies ileal atresia (8), thalidomide ingestion (9), or trisomy 18. Patients with trisomy 18 usually have multiple gastrointestinal and extragastrointestinal congenital abnormalities (6).
Positional Abnormalities
The appendix may lie in unusual locations, usually because of the cecal mobility, excessive appendiceal length, situs inversus, or intestinal malrotation. This may cause an otherwise typical appendicitis to present in atypical ways.
Duplications
Three patterns of appendiceal duplication exist: Double barreled, paired, and accessory (10). In “double-barrel” appendix, two separate tubes, each lined by a mucosa and separated by a submucosa, lie within a single muscular coat. Two symmetrically placed appendices lie on either side of the ileocecal valve in the paired form of duplication. This only occurs in infants with multiple congenital anomalies. A normal-appearing appendix lying in its usual position and a second rudimentary appendix arising from the cecum represents the accessory type of duplication. Triplication of the appendix can also occur (11).
Heterotopias
Heterotopic tissues rarely affect the appendix. However, heterotopic gastric, esophageal, ileal, and pancreatic tissues can all occur in this location.
Diverticulosis
Diverticula affect 0.004% to 2.8% of histologically examined appendices (5,12). They can be congenital or acquired; both types may be single or multiple. Congenital diverticula present as antimesenteric outpouchings with complete muscular walls, contrasting with acquired diverticula that lack the muscularis propria (Fig. 8.6). Some congenital diverticula are attached to the umbilicus by a fibrous band, resembling Meckel diverticulum.
Acquired diverticula are ten times more frequent than their congenital counterparts (5). They affect both sexes equally and develop along the area of the penetrating arteries, often secondary to inflammation or tumors. Associated neoplasms, particularly low-grade mucinous neoplasms, are present in many patients (Fig. 8.6) (13). Diverticula develop due to the increased pressure of the accumulated intraluminal mucin. A similar mechanism may be responsible for the relatively high incidence (14%) of appendiceal diverticula in patients with cystic fibrosis.
Acquired diverticula are commonly multiple (Fig. 8.6), lying along the mesenteric and antimesenteric borders. They usually involve the distal appendix, giving the appendix a beaded appearance. Their size varies from 2 to 5 mm. Like colonic diverticula, they are subject to inflammation or perforation. Inflammation may distort, obliterate, or disrupt a diverticulum. When the inflammatory process spreads into the periappendiceal tissues, an abscess results.
Appendiceal Intussusception, Autoamputation, and Inverted Appendiceal Stump
Appendiceal intussusception is rare, usually affecting young boys (14). Patients range in age from 8 months to 75 years. Predispositions to intussusception include the presence of a fetal cone-shaped appendix, an unusually thin mesoappendix, or the presence of mass lesions, most typically endometriosis, adenomas, carcinoid tumors, or the lymphoid hyperplasia associated with viral infections. The presenting signs and symptoms resemble those of acute appendicitis. The lesions may also remain asymptomatic only to be discovered incidentally.
TABLE 8.1 Types of Appendiceal Intussusception | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
There are four types of appendiceal intussusceptions (Table 8.1). In some cases, the distal appendix intussuscepts into the proximal appendix; in other cases, the proximal appendix intussuscepts into the cecoappendicular opening or the whole appendix intussuscepts into the cecum (Fig. 8.7), presenting as an edematous or infarcted cecal “polyp.” Intussusception can also appear as an umbilicated area at the junction of the taeniae coli on the cecal serosal surface. The mucosa may appear normal, hyperplastic, inflamed, eroded, or ischemic. The latter occurs if the vascular supply has been compromised. If there have been recurrent intussusceptions, the mucosa and muscular layers may become hyperplastic. In intussusception, the histology may also appear to be reversed from normal, with the epithelium lying on the external surface of tissue and the submucosa and muscularis propria lying inside the mucosa (Fig. 8.8). The submucosa becomes edematous and the muscularis propria may appear
hyperplastic, fibrotic, or splayed (Fig. 8.9). The muscular layers maintain a normal relationship with one another and with the submucosa and mucosa. Sometimes the bowel wall appears to have two muscularis propriae. It is important that the appendix be examined carefully in this setting to exclude the presence of an underlying neoplasm or other lesion such as endometriosis that may have been the lead point for the intussusception. Treatment of the intussusception is surgical resection.
hyperplastic, fibrotic, or splayed (Fig. 8.9). The muscular layers maintain a normal relationship with one another and with the submucosa and mucosa. Sometimes the bowel wall appears to have two muscularis propriae. It is important that the appendix be examined carefully in this setting to exclude the presence of an underlying neoplasm or other lesion such as endometriosis that may have been the lead point for the intussusception. Treatment of the intussusception is surgical resection.
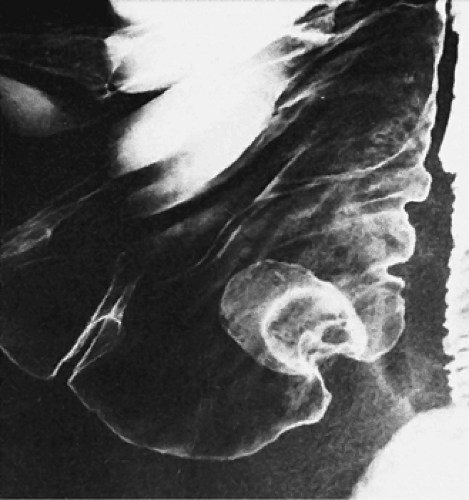 FIG. 8.7. Appendiceal intussusception. Double-contrast barium examination demonstrating inverted appendiceal stump. |
Occasionally, the appendix autoamputates following intussusception or volvulus. The presence of cecal scarring and hemosiderin in the absence of other cecal abnormalities provides clues that the appendix was present at birth. Inverted appendiceal stump follows appendiceal autoamputation, and may appear as a cecal polyp grossly or endoscopically. One of the complications of an appendiceal stump is a vascular malformation. Patients may present with massive bleeding from cecal ulcerations. Histologically, massively dilated vessels are present.
Torsion
Appendiceal torsion is rare, and when it occurs it causes ischemic appendicitis (Fig. 8.10). Histologically, one sees distal inflammation with areas of hemorrhage and necrosis. As with intussusceptions, a careful examination for the presence of an underlying neoplasm, as well as the assessment of the adequacy of the resection of the appendix if a neoplasm is present, is important.
Septated Appendix
Single or multiple, complete or incomplete septa consisting of mucosa and submucosa may divide in the appendiceal lumen into compartments (Fig. 8.11), predisposing the appendix to develop appendicitis. The inflammation usually remains confined to one compartment of the septated lumen. These lesions present most often in the 15- to 19-year age group with a clear-cut male predominance. They represent residual fetal septations.
“Absent Appendix”
The appendix may appear to be absent for several reasons: (a) agenesis; (b) previous resection; (c) obliteration from previous episodes of acute appendicitis, intussusception, or torsion; or (d) its presence in unusual locations. Retrocecal, retrocolic, and retroileal appendices are uncommon but can cause clinical confusion, especially in the individual who presents with acute abdominal pain. Acute appendicitis can resolve, leaving only a thin fibrous cord. Resection of the appendix should be suspected in individuals who have undergone previous surgical procedures. The confluence of the three cecal taeniae is the only consistent landmark for the appendiceal origin.
Appendicitis
Demographic Features
Appendicitis develops at any age, with a peak incidence in the 2nd and 3rd decades. However, it also affects neonates and the very elderly. Between 7% and 12% of the U.S. population develop appendicitis. Appendicitis occurs more commonly in Western cultures than in Eastern cultures, likely due to dietary differences between these populations (15). Heredity may also play a role in the pathogenesis of appendicitis (16). Appendicitis affects males more commonly than females, particularly during early childhood (17).
Acute appendicitis may also develop in neonates. Although this is rare, it associates with high morbidity and mortality rates (18,19). Neonatal appendicitis usually results from the presence of neonatal necrotizing enterocolitis, cystic fibrosis, Hirschsprung disease, or the bacteremia associated with maternal chorioamnionitis (18).
Despite the proclivity of appendicitis to involve younger individuals, it also affects the elderly and other age groups. The incidence of appendicitis in the elderly may be increasing due to their longer life expectancies. Appendicitis in the elderly also has a high mortality and complication rate (20), perhaps due to concomitant nonsteroidal anti-inflammatory drug (NSAID) use. NSAIDs may impair the inflammatory processes and suppress white cell responses increasing the risk of developing appendicitis (20). Additionally, NSAIDs mask the symptoms so that patients present with late-stage disease.
Pathophysiology
The etiology of appendicitis is multifactorial. It may involve obstruction, ischemia, infections, and hereditary factors. A common scenario in the pathogenesis of the disease involves a sequential series of events that begins with luminal obstruction due to any of the factors listed in Table 8.2. The obstruction is followed by loss of mucosal integrity, ischemia, and bacterial invasion. Secretions accumulate under pressure behind the obstruction. The mucosa can also be primarily involved by infections, as in the rest of the intestines without antecedent obstruction, or it may be affected by inflammatory bowel disease (IBD). Bacterial, viral, fungal, and parasitic diseases may all cause specific forms of acute appendicitis. However, microbiologic studies generally show that no single organ is identified; rather, a mixed aerobic and anaerobic bacterial population is present in most cases. The most commonly isolated bacteria are Bacteroides fragilis and Escherichia coli (21). Streptococcus milleri can also be detected (22). S. milleri may be more important than some of the other bacterial species, since these have a sevenfold increased risk for abscess formation (22). Campylobacter jejuni is also an important cause of acute appendicitis (23).
TABLE 8.2 Causes of Appendiceal Obstruction Leading to Appendicitis | |
|---|---|
|
Once an infection becomes established, pressure from inflammation and edema predisposes to the rapid development of gangrene, perforation, and peritonitis. Infections may also cause fibrin thrombi, which can block the small appendiceal vessels, causing secondary ischemia. The appendix is particularly prone to ischemia, since the appendiceal artery is an end artery. The enteric nervous system may also play a role in the pathogenesis of acute appendicitis. Increased numbers of nerve fibers, Schwann cells, and enlarged ganglia have all been found in patients with acute appendicitis (24).
Clinical Features
The diagnosis of appendicitis is straightforward when it presents classically with right lower quadrant abdominal pain of short duration, abdominal rigidity, and anorexia. Appendicitis also causes acute periumbilical, colicky pain or vomiting. Fever and leukocytosis develop early. However, there are many examples of appendices that are removed for suspected acute appendicitis in which histologic evidence of an acute appendicitis is lacking.
Pathologic Features
Normally, the appendiceal mucosa appears smooth, light yellow-tan; the serosa appears pink-tan, smooth, and glistening. When the inflammation is restricted to the mucosa, the exterior of the appendix may grossly appear normal. Dilation and congestion of serosal vessels produce localized or generalized hyperemia and constitute the earliest visible external changes (Figs. 8.12, 8.13, and 8.14). Well-developed acute appendicitis shows marked congestion with a dull (rather than glistening) serosal surface or there may be a serosal granular, fibrinous, or purulent coating and vascular engorgement reflecting severe necrosis and inflammation. The mesoappendix appears edematous and contiguous structures may become inflamed. The appendix often exudes purulent material from the cut surface; one may sometimes identify an impacted intraluminal fecalith. Mucosal necrosis and ulceration are usually present. The acute inflammation can localize to one segment of the organ or the entire appendix may be affected. There may be the appearance of a mucocele. If so, the appendix should be well sampled to exclude the presence of a coexisting mucinous neoplasm.
By the time full-blown gangrenous appendicitis develops, the organ appears soft, purplish, and hemorrhagic or even greenish black, sometimes with visible thrombi in the mesoappendix. These may spread along the ileocecal and upper mesenteric veins. Perforation may be present. In complicated cases, abscesses may form around a site of perforation and inflammation may extend into the mesoappendix (Fig. 8.15).
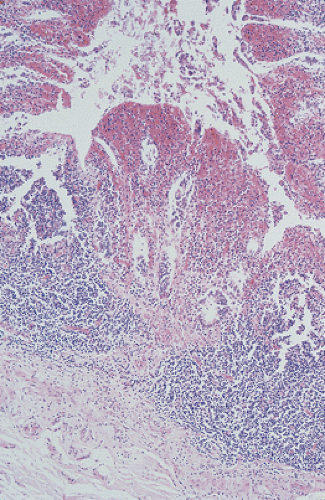
Histologic changes associated with appendicitis reflect disease duration and severity, and some changes may not reflect clinical disease at all. The term acute intraluminal appendicitis has been used when there are neutrophils in the appendiceal lumen but they have not yet infiltrated the mucosa (25). This may not be of any significance since this finding can be seen in incidental appendectomy specimens. Other minimal changes may consist of focal neutrophilic collections in the lumen and lamina propria. This is sometimes referred to as mucosal or early appendicitis. The term mucosal appendicitis has been used both in the presence and the absence of ulcers if the inflammation is restricted to the mucosa (26,27,28). The clinical significance of pure mucosal inflammation in the absence of ulcers is uncertain. Since these changes may reflect sampling error, more sections should be taken of the appendix to be certain that there is not more extensive inflammation elsewhere in the appendix.
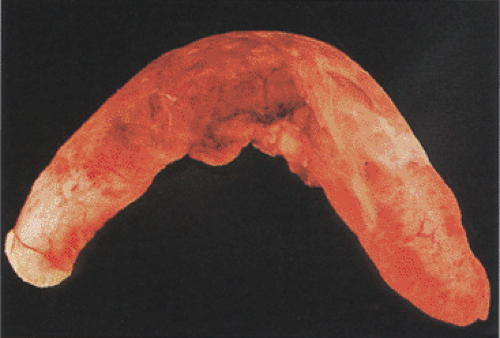 FIG. 8.13. Acute appendicitis showing vascular engorgement and serosal erythema. A white purulent membrane covers the serosal surface. |
In better-developed disease, focal erosions, cryptitis, and crypt abscesses develop. The inflammation then extends to the submucosa. After the inflammatory process reaches the submucosa, it spreads quickly to involve the remaining appendix. Eventually, the mucosa erodes, the wall becomes necrotic, and the vessels may thrombose. Submucosal abscesses, edema, and congestion follow. Some appendices contain prominent eosinophilic infiltrates. Extravasated mucin in the bowel wall may induce a foreign body reaction or even small mucin granulomas.
Gangrenous appendicitis shows extensive suppuration, often extending deep into or through the appendiceal wall with complete mural destruction (Figs. 8.16 and 8.17) with or without rupture. If perforation occurs, an intense nonspecific
inflammatory process ensues. Perforation may be suspected clinically, but it is sometimes difficult to see in the resected specimen due to the extensive inflammation. Resolving appendicitis is characterized by the presence of a predominantly lymphocytic infiltrate involving the subserosa and muscularis propria or the subserosa. When appendicitis heals, it assumes one of two basic patterns: The “usual” pattern, sometimes with an intraluminal cord of granulation tissue, and a xanthogranulomatous pattern (29). Fibrosis may develop.
inflammatory process ensues. Perforation may be suspected clinically, but it is sometimes difficult to see in the resected specimen due to the extensive inflammation. Resolving appendicitis is characterized by the presence of a predominantly lymphocytic infiltrate involving the subserosa and muscularis propria or the subserosa. When appendicitis heals, it assumes one of two basic patterns: The “usual” pattern, sometimes with an intraluminal cord of granulation tissue, and a xanthogranulomatous pattern (29). Fibrosis may develop.
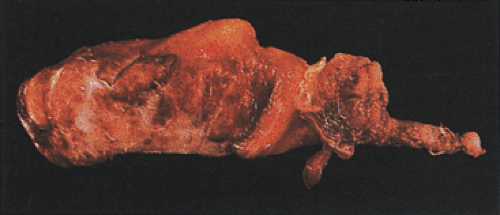 FIG. 8.14. Gangrenous appendicitis. The external surface of the appendix is hemorrhagic and reddened with a well-developed fibropurulent membrane. |
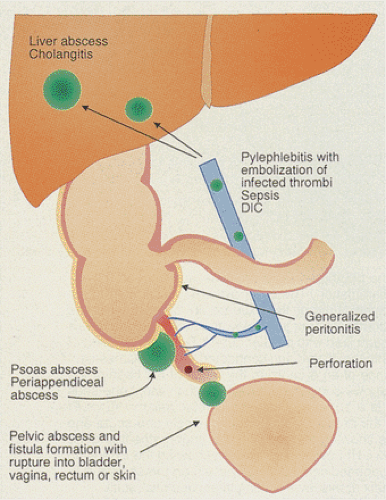 FIG. 8.15. Diagram illustrating the complications of acute appendicitis. DIC, disseminated intravascular coagulation. |
Although full-blown appendicitis is histologically easily identified by mucosal ulceration and neutrophilic infiltrates extending through the appendix wall to the peritoneal serosa, occasionally minimal inflammation can be difficult to diagnose due to inconspicuous pathologies. Staining with E-selectin, the first inducible cell adhesion molecule, expressed in early appendicitis, may help detect minimal or less obvious disease (30), although correlation between clinical findings and pathologic abnormalities may be speculative at best.
Complications
The most common complication of appendicitis is perforation, which can lead to generalized peritonitis, subdiaphragmatic and periappendiceal abscesses, serosal pneumatosis, and suppurative pylephlebitis (Fig. 8.15). Infected thrombi may involve the serosal and mesoappendiceal small vessels and may extend or embolize to distant sites such as the liver, where they can establish secondary bacterial infections, cholangitis, and hepatic abscesses (31). Fistulae may form between the appendix and the rest of the gastrointestinal tract, vagina, or bladder.
If a perforation occurs slowly, the inflammation often walls itself off, producing a periappendiceal abscess that typically localizes in the right iliac fossa lateral to the cecum. One may find little in the way of residual appendix and only a mass of granulation or xanthomatous tissue surrounding the abscess. This can progress to larger masses of chronic fibrous tissue containing granulomas. Extensive granulomatous reactions may raise the clinical suspicion of a cecal carcinoma. These granulomas often contain foreign material including feces. In some cases, the inflammatory reaction results in mesothelial entrapment that eventually becomes cystic, simulating a benign cystic mesothelioma, particularly if the mesothelial lining appears reactive and atypical.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree