Non-CMV, Non-hepatitis Viral Infections in the Renal Transplant Patient
Emilio Ramos
Michelle Josephson*
Division of Nephrology, University of Maryland School of Medicine, Baltimore, Maryland 21201; and *Section of Nephrology, University of Chicago School of Medicine, Chicago, Illinois 60637
INTRODUCTION
The past decade has witnessed enormous progress in the field of transplantation. This has been characterized by such events as the development of new therapeutic molecules through recombinant technology to diagnostic technologies such as polymerase chain reaction (PCR). In addition, new immunosuppressive agents have appeared, including calcineurin inhibitors, mycophenolate mofetil, sirolimus and the interleukin (IL)-2 antagonist antibodies.
With such advanced technologies and the advent of more potent and effective immunosuppressive agents, we have seen a remarkable improvement in 1-year graft survival in patients with renal transplantation, exceeding 90% in most transplant centers. This has been accompanied by an increase in survival. Patients are able to conduct their lives with regard to employment, travel, and activities in a manner that was not possible prior to transplantation.
In this chapter, we describe some of the viral infections seen in the renal transplant population. Some of the viruses are transmitted by the organ itself, such as polyomavirus, parvovirus, West Nile virus, Epstein-Barr virus (EBV) and human immunodeficiency (HIV). In rare instances, the virus is in a latent stage and is later reactivated with intense immunosuppression, such as in patients with Polyoma virus, herpes simplex virus (HSV), EBV, human herpes virus (HHV)-6 and HHV-8. We have also seen the emergence of new pathogens in immunocompromised hosts, including BK virus, JC virus, HHV-6, HHV-7, HHV-8, ganciclovir-resistant cytomegalovirus (CMV) and West Nile virus. In addition to unusual manifestations in BK virus nephritis, we have also seen the presence of viral co-infections common in these patients like CMV, chronic rejection, EBV and posttransplant lymphoproliferative disorders (PTLD). Viral infections can also modulate the activation of leukocytes and endothelial cells, as well as the major histocompatability complex antigen influencing rejection. It is also evident that many of these viral infections can modulate the process of oncogenesis.
We discuss several aspects of these viral infections, including pathogenesis, diagnosis and current available treatments in this chapter.
POLYOMAVIRUS
Overview
In 1970, virologist Sylvia Gardner and colleagues identified a new polyomavirus (1,2). The virus was isolated from a 39-year-old Sudanese male (with initials B.K.) who presented to St. Mary’s Hospital in London with anuria secondary to ureteral obstruction. This individual had received a living related donor transplant from his brother approximately 3.5 months earlier. The virus was named B.K. after the patient from whom it was isolated.
At about the same time, JC virus, a polyomavirus associated with Progressive Multifocal Encephalopathy (PML), was isolated and like B.K. it was named after the individual from whom it was isolated (2). Both BK and JC are human polyomaviruses and both were first described in the same 1971 Lancet publication (2). Polyomaviruses were previously classified as a subfamily of the Papovaviridae, along with papillomaviruses. Polyomaviruses are no longer considered a Papovaviridae subfamily and now make up their own genus (3). Polyomaviruses are small nonenveloped viruses with covalently closed, circular double-stranded DNA genomes (4). The capsid has icosahedral symmetry and is made up of 72 capsomeres (4). Polyomaviruses are 40nm to 45nm in diameter (4).
Polyomaviruses are ubiquitous. They are found in parakeets, athymic rats, mice, hamsters, rabbits, cattle, monkeys as well as humans (4). Monkeys can be infected with several different polyomaviruses (4). Although the viruses are species specific (4), through the interventions of modern medicine and technology humans have been exposed to SV40 (a monkey polyomavirus) (5, 6, 7). The cross-species exposure occurred when the polio vaccine was prepared. Rhesus monkey kidney cells were used to produce both inactivated and live-attenuated polio vaccine. Some of the kidney cells were harvested from monkeys infected with SV40 (a virus that was not recognized at the time). The virus survived vaccine inactivation. From 1955 to early 1963, millions of people were exposed to live SV40-contaminated poliovirus (5). And from 1961 to 1965, military and civilians were exposed to SV40 when receiving adenovirus vaccines (5). The prevalence of SV40 infection in the human population is unknown (5).
Primary infection with BK virus occurs as a mild respiratory infection in young children (3,8,9). In the United States, antibodies to the BK virus are present in 50% of 3 to 4 year olds and nearly 100% of children have antibodies by 10 to 11 years (4,10). Adult levels of seroprevalence are 65% to 90%. There appears to be a decrement of antibody positivity in adulthood (3,9,11). Maternal antibody is found in neonatal blood but is lost within the first few months of life. After the initial infection, it is hypothesized that the virus persists as a latent infection predominantly in the kidney. BK and JC virus DNA have been isolated from the kidneys of healthy individuals supporting this idea (12, 13, 14). PCR has demonstrated BK virus sequences in many tissues including liver, stomach, lungs, parathyroid glands, and lymph nodes (15). Transmission of the virus is not well understood though it likely occurs through exposure to body fluids (10,12). Reactivation occurs during periods of immune impairment such as therapeutic immunosuppression, HIV infection, lymphoproliferative disease, systemic lupus erythematosus (SLE), pregnancy, diabetes, and aging (4).
Clinical Manifestation of Polyomavirus-associated Nephropathy
BK reactivation presents with shedding of the virus in the urine in the form of decoy cells in 10% to 68% of patients (16,17). Many of these patients are asymptomatic; however, as in the first case described in 1971, BK reactivation can present with ureteric inflammation (1), ulceration and fibrosis leading to obstruction. In the pre-calcineurin inhibitor era, most patients presented with either the presence of asymptomatic viruria or, in some cases, presented with ureteric stenosis and obstruction. Following the advent of new and more potent immunosuppressive agents, like calcineurin inhibitors and mycophenolate mofetil, improved surgical technique and the use of stents in cases of stenosis, the pattern of clinical presentation of BK replication has changed. Patient now presents with either asymptomatic viruria or with renal dysfunction (18, 19, 20). Renal dysfunction is manifested in the form of acute renal failure or a chronic renal failure, with characteristic histological changes in kidney biopsy (21). Polyomavirus-associated nephropathy (PVAN) manifested by characteristic histological features and renal dysfunction is found in about 1% to 8% of renal transplant patients (22,23). In our own study (24) which included 96 patients with PVAN documented by renal biopsy, the diagnosis was made within a mean time of 14.4 months (1.2 to 53 months following transplantation); however, clinical manifestations less frequently seen are ureteric stenosis as in the first case described in 1971, cystitis, lymphocele, hydronephrosis and urinary tract infection. Persistent PVAN is associated with a high and irreversible incidence of allograft failure, varying between 12% and 100%; mean 30% over a 10 to 240 week follow-up (24). In our cohort study (24), the graft loss was 30%. In addition, 30% of the patients had a serum creatinine greater than 3 mg/dl (Fig. 27.1). Of interest, eight patients had acute cellular rejection concurrent with PVAN at the time of diagnosis. Those patients were treated with intravenous prednisone followed by decreased immunosuppression. In addition, four patients had concurrent clinical and histological evidence of CMV infection, which was effectively treated with ganciclovir. The mean age was 53.4 years, range 12 to 79. As had been reported previously (25), patients with PVAN were significantly older when compared with patients negative for PVAN (p = 0.001). Forty transplants were from living donors and 56 were from cadaveric donors. There was no difference in the incidence of PVAN in patients who received a living versus a cadaveric donor. In addition, there
was no difference in the incidence of PVAN in patients with prior incidence of delayed graft function (DGF) or acute rejection compared to a control group of patients with similar renal function transplanted at the same time but negative for PVAN on urine cytology.
was no difference in the incidence of PVAN in patients with prior incidence of delayed graft function (DGF) or acute rejection compared to a control group of patients with similar renal function transplanted at the same time but negative for PVAN on urine cytology.
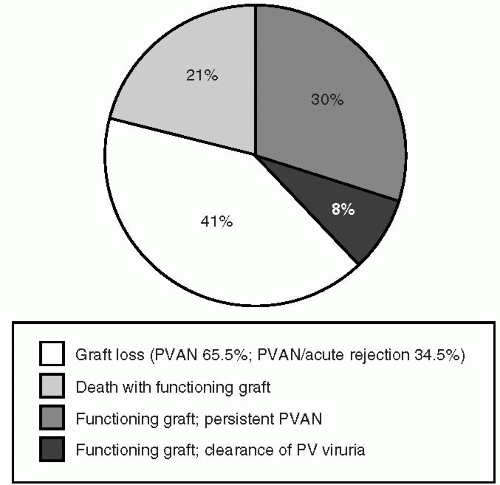 FIG. 27.1. Graft outcomes in patients with BK-associated nephritis. (From Cecka JM, Terasaki PI, eds. Clinical Transplantation. Los Angeles, CA: UCLA Immunogenetics Center, 2002, with permission.) |
BK Virus in Nonrenal Transplant Patients
Polyoma viruria (JC as well as BK) has been observed in patients receiving chemotherapy (25) and has been associated with hemorrhagic cystitis after bone marrow transplant (26, 27, 28, 29). Viruria has been noted in patients with SLE (30), patients with HIV (31, 32, 33), pregnant women (JC more than BK) (34,35), heart transplant recipients (36), pancreas alone recipients (37) and diabetics (16).
Like viruria, nephropathy has also been observed in nonrenal transplant patients (37). Reported cases include a 6-year-old boy with hyperimmunoglobulin M deficiency (38), a child with cartilage-hair hypoplasia and Hodgkin’s disease (39), patients with HIV (40, 41, 42, 43, 44, 45), two patients with Chronic lymphocytic leukemia (CLL) (35,36), (both of whom had both CLL and BK in their kidneys) and a patient with a solitary pancreas (46). The common theme uniting these cases with the renal transplant patients is that all of these patients were immunosuppressed.
Diagnosis of Polyomavirus-associated Nephropathy
The diagnosis of PVAN can be made using either invasive or noninvasive techniques.
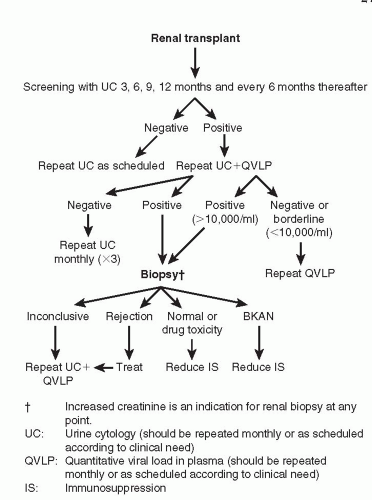
Noninvasive techniques. The presence of infected decoy cells in the urine in patients with BK reactivation detected using urine cytology has proven to be extremely useful clinically (17,20). It is an inexpensive technique, widely available and an excellent source for screening and surveillance in the evaluation of BK infection. Urine cytology with the presence of decoy cells detected in the urine often precedes the diagnosis of PVAN, and cells cease to exfoliate in the urine when the BK virus has been cleared or in the late stages of PVAN when the graft biopsies show extensive scarring. We routinely use urine cytology for surveillance in our patients following transplantation. It is important to differentiate between transient and persistent exfoliation of decoy cells. Transient exfoliation is seen anytime tubular injury occurs, as in cases of acute tubular necrosis, delayed graft function and with initiation of angiotensin-converting enzymes (ACE) inhibitors. Patients who have persistent BK viruria, however, have a high probability of developing PVAN even with normal initial renal function (24). Quantitative PCR of the urine is also used (48) although it is much too sensitive and, as such, not very useful for clinical purposes (22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50). In addition, measurement of BK viral load in plasma correlates with PVAN. In a prospective study, Hirsch et al (22) showed that patients with PVAN have a plasma viral load that was statistically significantly higher (p<0.001) when compared to patients without PVAN. In that study, patients with PVAN had more than 7,700 BK virus copies per mL of plasma. In comparing the two methods of urine cytology and BK viremia, the predictive value of urine cytology is 29% versus 50% in patients with BK viremia. The specificity was 71% for urine cytology and 88% for quantitative BK PCR viremia. Interestingly, both methods have a negative predictive value of 100%. Many transplant centers, including the University of Maryland (24), have developed management algorithms based on the presence or absence of BK DNA in both plasma and urine as a way to assess the effect of decreased immunosuppression. Typically, based on the results of urine cytology or the quantitative PCR plasma levels, the antimetabolite is decreased or withdrawn. If, following this, urine or blood continues to be positive after 3 weeks, calcineurin inhibitors are usually decreased next (Fig. 27.2).
Invasive techniques. A gold standard for diagnosing BK-associated nephropathy is the renal biopsy. Histological changes are quite characteristic and have been described previously (12,20,24). At the onset, even with the renal function within normal limits we see a variable degree of inflammatory cells, mainly lymphocytes and plasma cells with viral cytopathic changes such as intranuclear viral inclusion in the tubular cells. The process usually starts with the cytopathic changes in the epithelial cells without much cellular
infiltration; then the cellular infiltration becomes sparse and more diffused consistent with the diagnosis of interstitial nephritis. Finally, there is fibrosis and sometimes calcification seen by histology. Immunostaining with SV40 virus also shows the presence of viral particles within the intranuclear cells. Rhandhawa et al (51) recently performed quantitative PCR of microdissected biopsy material and found low levels of BK virus in biopsies preceding the diagnosis of PVAN. The quantitative PCR increased markedly at the time of development of PVAN. This method may indeed also prove useful in recognizing patients at risk for developing PVAN.
infiltration; then the cellular infiltration becomes sparse and more diffused consistent with the diagnosis of interstitial nephritis. Finally, there is fibrosis and sometimes calcification seen by histology. Immunostaining with SV40 virus also shows the presence of viral particles within the intranuclear cells. Rhandhawa et al (51) recently performed quantitative PCR of microdissected biopsy material and found low levels of BK virus in biopsies preceding the diagnosis of PVAN. The quantitative PCR increased markedly at the time of development of PVAN. This method may indeed also prove useful in recognizing patients at risk for developing PVAN.
Impact on Graft Function and Graft Failure
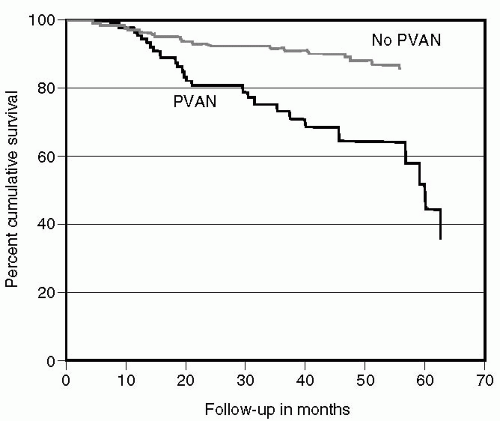
BK nephropathy causes allograft dysfunction and if left unchecked reduces graft survival (20,24). Randhawa et al (52) identified 22 cases of BK nephropathy. Immunosuppression was reduced in 8, antirejection therapy was administered to 12, and 2 were diagnosed at the time of nephrectomy. Ten of the 14 who did not undergo immunosuppression reduction lost their allografts. Nickeleit et al (53) also demonstrated a poor outcome in patients with BK nephropathy. Graft loss occurred in 5 of 11 patients diagnosed with BK nephropathy within an average of 6 months of diagnosis. Ramos et al (20) compared graft survival in patients undergoing kidney biopsy for allograft dysfunction. Allografts with dysfunction secondary to BK nephropathy had a significantly shorter mean graft survival than those with dysfunction secondary to other causes (Fig. 27.3).
Risk Factors
Risk factors for nephropathy are poorly defined. Unlike the case for CMV, it is not clear that primary infection posttransplant is of more concern than reactivation. For example, it has not been demonstrated that BK seronegative recipients of BK positive kidneys are at greater risk for the development of BK nephropathy than seropositive recipients (22). Given the high rate of seropositivity (60% to 80%) and relatively low rate of nephropathy (1% to 8%) (20), it seems likely that specific risk factors do promote the transformation of latent virus into active disease. Several risk factors have been implicated, although there is no consensus. Nickeleit et al found cellular rejection occurred more commonly in patients with BK nephropathy than controls (54). High-dose immunosuppression (55), tacrolimus (54,55) and mycophenolate mofetil (54), male gender (20), older age (20), tubular injury (through rejection or other means) (51), exposure to antilymphocyte therapy or increased corticosteroid pulses (22,55), an increased number of human leukocyte antigen (HLA)
mismatches between donor and recipient, cadaver renal transplants (22,55), and presence and degree of viremia (22) have been named as potential culprits. By contrast, other researchers have not been able to confirm that use of specific immunosuppressive agents (20,22) or administration of antilymphocyte antibodies (22) predisposes to BK nephropathy. The rarity of BK nephropathy in immunosuppressed recipients of nonrenal organs is most likely due to one or both of the following possibilities: (a) that we have overlooked or failed to consider it, and/or (b) that, in order for a patient to develop BK nephropathy, renal injury (occurring for example during rejection or warm ischemia/reperfusion) may be required as a cofactor (55,56).
mismatches between donor and recipient, cadaver renal transplants (22,55), and presence and degree of viremia (22) have been named as potential culprits. By contrast, other researchers have not been able to confirm that use of specific immunosuppressive agents (20,22) or administration of antilymphocyte antibodies (22) predisposes to BK nephropathy. The rarity of BK nephropathy in immunosuppressed recipients of nonrenal organs is most likely due to one or both of the following possibilities: (a) that we have overlooked or failed to consider it, and/or (b) that, in order for a patient to develop BK nephropathy, renal injury (occurring for example during rejection or warm ischemia/reperfusion) may be required as a cofactor (55,56).
Treatment of Polyomavirus-associated Nephropathy
Currently, the most accepted treatment for PVAN is reduction of immunosuppression (24,25). The reduction in immunosuppression is done monitoring carefully both the urine cytology and the plasma levels (PCR). Typically, one starts by withdrawing the antimetabolite and, if the viremia or urine cytology does not decrease significantly, the calcineurin inhibitors are reduced. At the University of Maryland, we aim at target levels of FK506 and cyclosporine of 6-8 ng/ml and 75-100 ng/mL, respectively (20). In some cases, in addition to the withdrawing of the metabolite, the calcineurin inhibitors are replaced by sirolimus with a target dose of 10-12 ng/mL (24). It is important in following these maneuvers that renal function, calcineurin levels and sirolimus levels are followed on a weekly basis to monitor improvement or deterioration of renal function. Deterioration of renal function should prompt a renal biopsy to rule out the presence of acute rejection or progression of PVAN. At University of Maryland, in cases where acute rejection is diagnosed concomitantly with PVAN, patients are treated with a 3-day pulse of Solu-Medrol consisting of 500 mg daily for 3 days, and prednisone is tapered rapidly over 3 weeks to a level of 5% to 10 mg%. Then, we start decreased immunosuppression as stated above.
At the present time, there is no Food and Drug Administration (FDA)-approved antiviral treatment for PVAN. Several antiviral compounds including cytosine, arabinoside, vidarabine (57,58) and amantadine (59) have been tried without much effect. Cidofovir, a cytosine phosphonate analog has been shown to have antipolyomavirus activity in vitro (60). It is renally excreted and has the potential of being nephrotoxic. Low-dose cidofovir, at a dose of .25 to 1 mg/kg every 2 weeks has been reported from a few centers with varying results (61, 62, 63). Although amelioration of BK viruria and viremia has been observed in most cases, some patients have had improvement in renal function whereas in others the renal function has continued to deteriorate, necessitating renal replacement therapy.
Retransplantation in Patients with Graft Loss Due to Polyomavirus Nephropathy
Since PVAN is an indolent infection, viral reactivation occurs with the use of immunosuppression therapy. It is important, then to consider whether or not these patients are suitable for retransplantation and, if so, whether we should alter their immunosuppressive therapy. We reported the first case of successful retransplantation of a patient who lost her kidney allograft to BK nephropathy. The patient is now more than 7 years out from her second transplant, with no evidence of BK virus recurrence and stable graft function (64). A more extensive evaluation of the role of retransplantation was done using data from five different U.S. transplant centers. We recently reported on 10 patients who were retransplanted after losing their original graft to BK nephropathy (65). All the patients were males and the median age was 43.5 years (mean 45.3, ± 12 months). In each case, immunosuppression was restarted after the second transplant, using the same dosage as used in the first transplant. All patients were on mycophenolate mofetil and prednisone in combination with tacrolimus (six patients), cyclosporine (three patients) and tacrolimus and azathioprine (one patient). After graft loss, transplant nephroureterectomy was performed in a total of seven patients. Five patients had this procedure several weeks prior to retransplantation, whereas two patients had the procedure done at the time of retransplantation. In three patients, nephroureterectomy was not performed. At the time of retransplantation, all 10 patients had the absence of viruria in urine cytology. Retransplantation was performed after a median period of 8.5 months. Follow-up after retransplantation occurred within a median period of 28.5 months. In this retrospective study, only 1 patient had recurrence of PVAN following retransplantation. The other nine patients have had good renal function, with a mean serum creatinine of 1.4 mg% after a mean follow-up of 31.5 months. Neither nephroureterectomy of an infected graft prior to retransplantation nor selection of a different immunosuppression regime appears to keep it the same Polyoma Virus Associated Nephropathy (PVAN). From the present study, we might conclude that patients with graft loss secondary to BKAN can be relatively safely retransplanted using standard immunosuppressive regimes. In addition, it is very important that patients who are considered for retransplantation have a negative urine cytology and absent or low serum BK viral load prior to retransplantation. It is, however, important to keep active surveillance for BK reactivation after kidney transplantation, using urine cytology and quantitative plasma viral load in those patients who are being retransplanted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree