Chapter 4.5
Non-alcoholic fatty liver disease and hereditary haemochromatosis and nutrition
Niamh O’Sullivan1 and Catherine McAnenny2
1St Vincent’s University Hospital, Dublin, Ireland
2Royal Infirmary of Edinburgh, Edinburgh, UK
4.5.1 Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is the liver manifestation of metabolic syndrome. The National Institutes of Health (NIH) define metabolic syndrome as having at least three of the following risk factors: increased abdominal girth, increased triglyceride concentrations, low high-density lipoprotein (HDL), high blood pressure and high fasting blood glucose. It is estimated that 48–100% of people with NAFLD are asymptomatic. Many have non-specific symptoms such as fatigue and right upper quadrant pain [1]. NAFLD is often an incidental finding from abnormal liver function tests, predominantly alanine aminotransferase (ALT). Often the ratio of aspartate aminotransferase (AST) to ALT is <1, which differentiates NAFLD from alcohol-related fatty liver disease.
The NIH Clinical Research Network on NAFLD has agreed that the maximum allowable level of alcohol intake for definition of NAFLD as opposed to alcoholic fatty liver disease is 140g ethanol per week for men and 70g for women. Practice guidelines from the American Association for the Study of Liver Disease (AASLD) recommend that ongoing or recent alcohol consumption of >21 units/week for men and >14 units/week for women is a reasonable definition for significant alcohol consumption when evaluating patients with suspected NAFLD in clinical practice [2].
Non-alcoholic fatty liver disease is histologically subcategorised into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). NAFL is defined as the presence of hepatic steatosis with no evidence of hepatocellular injury in the form of ballooning of the hepatocytes. NASH is defined as the presence of hepatic steatosis and inflammation with hepatocyte injury with or without fibrosis [2]. Figure 4.5.1 presents the stages of NAFLD.
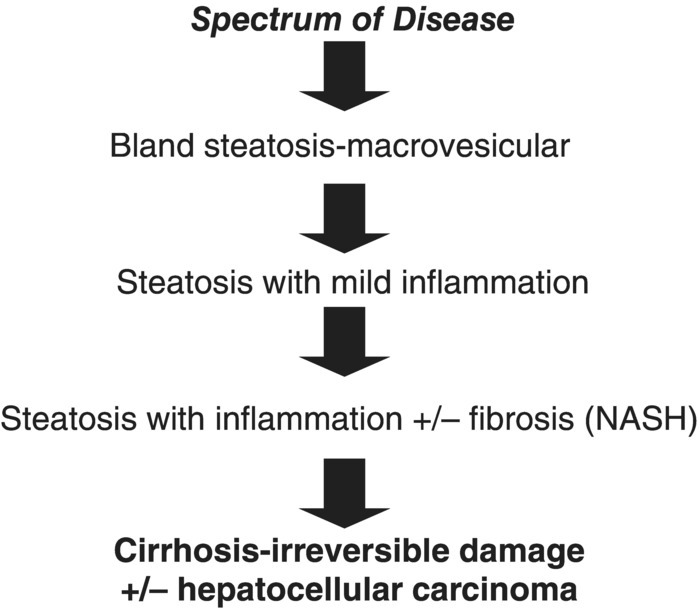
Figure 4.5.1 Stages of non-alcoholic fatty liver disease.
Approximately 5% of people with NAFLD develop end-stage liver disease. Mortality is greater than in age- and gender-matched controls [1]. Most cryptogenic cirrhosis and 25% of liver disease are caused by NAFLD [3,4]. Liver disease is the third most common cause of death in NAFLD [1].
The prevalence of NAFLD increases with age [5]. Estimates of worldwide prevalence of NAFLD range from 6.3% to 33% with a median of 20% in the general population [2]. In America the prevalence of NAFLD is 17–33%, whilst in Europe it is estimated at 20–30% [5,6]. The estimated prevelance of NASH is lower and ranges from 3% to 5% [2].
Non-alcoholic fatty liver disease is linked with insulin resistance, diabetes, hypertension and obesity, particularly central obesity (Table 4.5.1). It affects 76% of obese individuals but NASH is only present in 18.5% of obese individuals whilst 80% of those with NAFLD are morbidly obese [1]. Only 3% of people with NAFLD have a normal Body Mass Index (BMI) but this subgroup does exhibit central obesity or insulin resistance [7]. In type 2 diabetes, rates of NAFLD are approximately 50–69% [1,8].
Table 4.5.1 Waist circumference levels for central obesity
| Waist circumference | ||
| Country/ethnic group | Male | Female |
| South Asian/ Chinese/ South and Central American/ Japanese | >90 cm | > 80 cm |
| European | >94 cm | >80 cm |
| USAa | >102 cm | >88 cm |
aATP III Adult Treatment Panel III values are used for clinical purposes.
Reproduced with permission from the World Health Organization.
Causes of non-alcoholic fatty liver disease
The cause of NAFLD is multifactorial, including genetic predisposition, lack of exercise, increased energy intake, obesity and insulin resistance (Table 4.5.2). The consumption of trans fats is associated with the development of NAFLD and hepatic inflammation and saturated fat intake is a risk factor for NASH in the obese as it increases insulin resistance [11]. Abdominal or central obesity increases the flux of free fatty acids to the liver. An overabundance of circulating fatty acids increases insulin resistance and in NAFLD there is no insulin-mediated suppression of lipolysis. The consumption of high-fructose corn syrup contributes to insulin resistance and NAFLD [12].
Table 4.5.2 Causes of non-alcoholic fatty liver disease
| Primary NAFLD | Secondary NAFLD (absence of insulin resistance) |
| Central obesity Insulin resistance Type 2 diabetes | Total parenteral nutrition Fatty liver of pregnancy Intestinal jejunoileal bypass surgery Post gastrointestinal surgery for obesity Metabolic conditions Medications |
Secondary NAFLD/NASH is rare in adults and is unrelated to insulin resistance or metabolic syndrome. Figure 4.5.2 illustrates the first- and second-hit hypothesis in NAFLD.
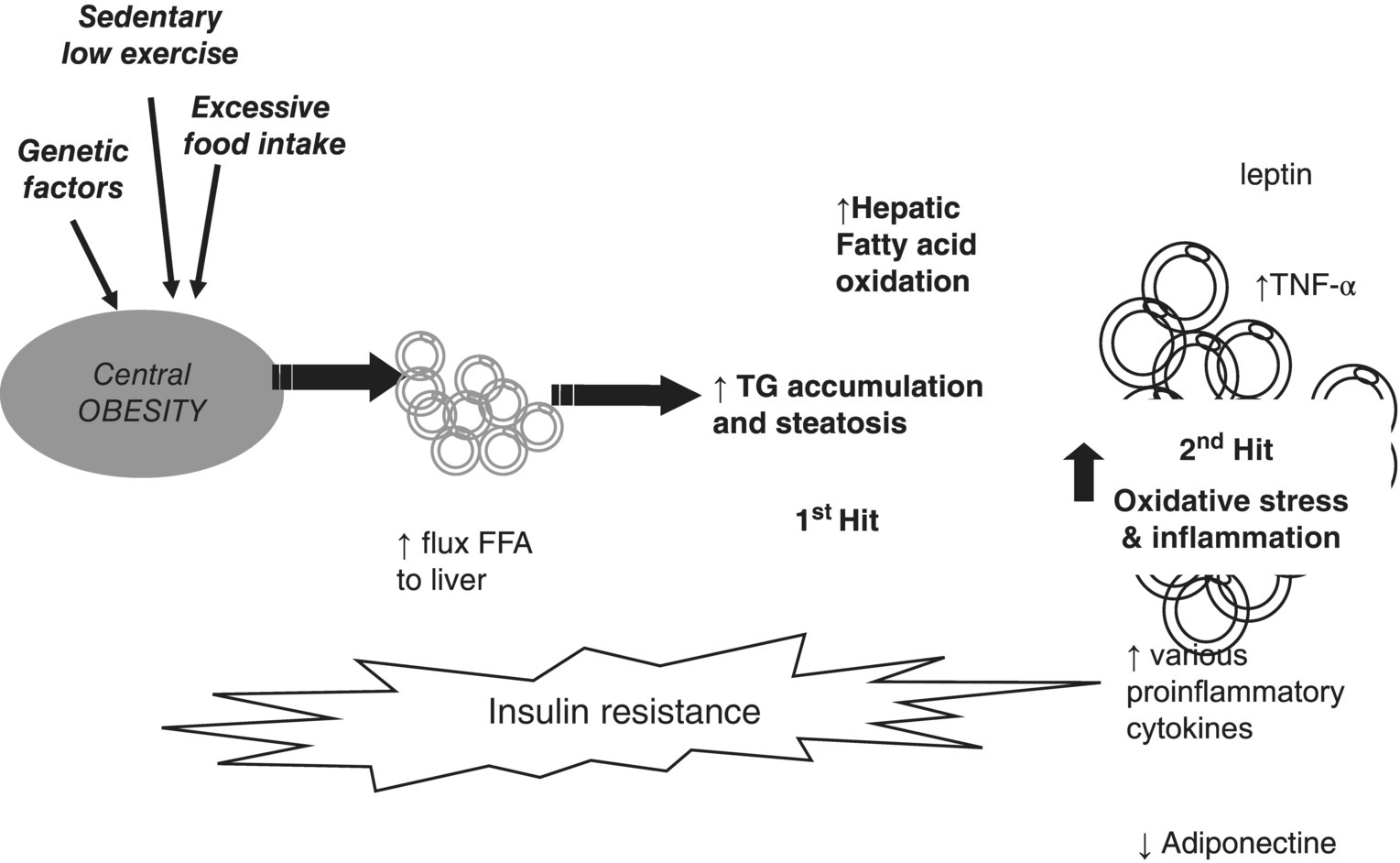
Figure 4.5.2 Two-hit theory of obesity-related hepatic fibrosis. FFA, free fatty acids; TG, triglyceride; TNF, tumour necrosis factor.
Nutritional assessment
A nutritional assessment of a patient with NAFLD should include weight, BMI, HDL, low-density lipoprotein (LDL), triglycerides, waist circumference, diet history and HbA1c if diabetic. BMI and waist circumference have both been shown to correlate with insulin resistance. Waist circumference also correlates with ALT concentrations. The presence of NASH with fibrosis is associated with being overweight and an increase in waist circumference [13].
Dietary management
Given the strong association between insulin resistance and NAFLD, it is reasonable to recommend lifestyle modification to all patients with NAFLD [14]. This decreases the risk of developing type 2 diabetes but an intense dietary intervention may also improve liver histology in people with NAFLD (Table 4.5.3) [15]. The present gold standard for the management of NASH is modest weight reduction, and a decrease in central obesity by combining dietary advice with increased physical activity [16,17]. Weight loss generally reduces hepatic steatosis, achieved either by hypocaloric diet alone or in conjunction with increasing physical activity [2,16]. Loss of at least 3–5% of body weight appears necessary to improve steatosis, but a greater weight loss (up to 10%) may be needed to improve necroinflammation [2]. Emphasis should be on decreasing abdominal girth [17]. Crash dieting should be avoided (weight loss greater than 1 kg/week) as it is associated with worsening liver function test abnormalities, accelerated fibrosis and exacerbated steatosis [1]. Patients should be monitored for subacute NASH during rapid weight loss [18].
Table 4.5.3 Potential beneficial effect of diet-induced weight loss on non-alcoholic fatty liver disease
| Main effect | Result |
| Reduced hepatic FFA supply | ↓TAG synthesis ↓Hepatic insulin resistance ↓Hepatic glucose output ↓ROS generation ↓Hepatocyte inflammation |







