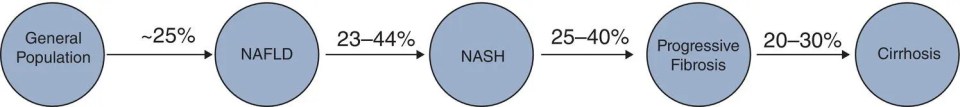
Matthew Collins1,3 and Keyur Patel2 1McMaster University, Hamilton, Ontario, Canada 2Toronto General Hospital, Toronto, Ontario, Canada 3Liver Care Canada, Hamilton, Ontario, Canada The term NAFLD and its clinical spectrum was first used 20 years ago and has since been accepted and increasingly referenced in clinical practice. NAFLD has been found to be strongly associated with metabolic disease, and rising prevalence parallels global increased rates of obesity and coinciding metabolic disease. NAFLD is now the most common chronic liver disease and the second most common indication for liver transplantation in developed nations. NAFLD is defined as hepatic steatosis, as evidenced by radiologic or histologic examination, in the absence of secondary causes of hepatic fat accumulation (Box 17.1). The definition of significant alcohol consumption varies between different society guidelines. The American Association for the Study of Liver Diseases defines significant alcohol consumption per consensus meeting recommendations for clinical trial candidate eligibility. Consumption of more than 21 standard drinks/week in men and more than 14 standard drinks/week in women over a two‐year period preceding baseline liver histology is considered significant; one standard drink contains 14g ethanol [1]. The European Association for the Study of the Liver defines significant alcohol consumption as daily alcohol consumption of 30 g or more for men and 20 g or more for women [2]. The term NAFLD encompasses the entire spectrum of disease ranging from uncomplicated simple hepatic steatosis (NAFL) to non‐alcoholic steatohepatitis (NASH) and cirrhosis. NASH is a histologic diagnosis and differentiating NASH from NAFL requires a liver biopsy. At the current time, NASH cannot be diagnosed accurately with imaging or blood work. NASH is characterized by the presence of 5% or more hepatic steatosis with inflammation and hepatocyte ballooning, with or without fibrosis. The presence of NASH is associated with progression to advanced fibrosis, cirrhosis and its complications, whereas the risk of developing advanced fibrosis is minimal in NAFLD [1]. Although most patients with NAFLD have an elevated body mass index (BMI), 10–15% of patients with NAFLD have normal BMI; this is defined as lean NAFLD, which appears to be more prevalent in patients of Asian descent. Recently, the term metabolic dysfunction‐associated fatty liver disease (MAFLD) has been proposed to replace NAFLD, to more appropriately reflect the association with metabolic disease and move away from NAFLD as a diagnosis of exclusion, and to remove alcohol from the definition. New “positive” criteria have been proposed for the diagnosis of MAFLD and include evidence of hepatic steatosis in addition to one of the following three criteria: elevated BMI/obesity, T2DM, or evidence of metabolic dysregulation [3]. The global prevalence of NAFLD is estimated to be approximately 25% [4]. Prevalence is higher in Asia Pacific, Latin America, and the Middle East. The lowest prevalence is observed in Africa. The prevalence is also increased in high‐risk groups (i.e. those with metabolic syndrome), as will be discussed later. Lean NAFLD is more common in Asian populations, where its prevalence has been reported at 19% [5]. Estimates on the prevalence of NASH are limited by the requirement of a liver biopsy for diagnosis. From the available data, the prevalence is estimated to be 1.5–6.45% in the general population. The prevalence of NASH in the NAFLD population ranges from 7% to 30% [4, 6]. In Canada, the prevalence of NAFLD is projected to increase by 20% between the year 2019 and 2030, with an even greater increase in cases of advanced fibrosis and the associated liver‐related complications including hepatocellular cancer [7]. The liver plays a central role in human metabolism and the disposal or storage of excess metabolic energy. NAFLD is thought to reflect the hepatic manifestation of the metabolic syndrome. Visceral adipose tissue makes up a small percentage of total body fat, but is highly metabolically active, and plays a significant role in free fatty acid delivery to the liver. The accumulation of fatty acids in hepatocytes is the hallmark of NAFLD. This results from multiple interacting pathways involving genetics, comorbidities, microbiome, and lifestyle (nutrition/behavior), but a key contributing factor is insulin resistance. The accumulation of free fatty acids in the liver leads to hepatocyte stress and inflammation, which activates stellate cells and leads to progressive fibrosis. Interestingly, as fibrosis worsens in NAFLD, biopsy features of steatosis and hepatitis often improve, and this is referred to as “burned out” NAFLD. Over the years, multiple risk factors for the development and progression of NAFLD have been identified. Perhaps the biggest risk factors are elevated intra‐abdominal or visceral fat and metabolic syndrome (three or more of: waist circumference ≥ 89 cm in women, 102 cm in men; high‐density lipoprotein < 1.3 mmol/l in women, 1.04 mmol/l in men; triglycerides ≥ 1.7 mmol/l; blood pressure ≥ 130/85 mmHg; fasting blood glucose ≥ 5.6 mmol/l). Studies have demonstrated that approximately 75% of patients who are overweight and 90–95% of patients with morbid obesity have NAFLD [8]. Likewise, 50% of patients with NAFLD and 90% of patients with NASH are likely to have metabolic syndrome [9]. BMI is a simple calculation and is commonly used in clinical practice to assess for obesity. A BMI ≥ 25 kg/m2 or > 23 kg/m2 in Asian populations is considered overweight, and a BMI > 30 kg/m2 or > 27 kg/m2 in Asian populations is diagnostic of obesity. However, this metric does not perform well at the extremes of height and in those with a high muscle mass. It also does not distinguish between subcutaneous and visceral fat. Waist circumference is considered a better indicator of intra‐abdominal or visceral fat, and has a greater association with metabolic disease than BMI. The UK National Institute for Health and Care Excellence suggests that men with a waist circumference greater than 94 cm and women with a waist circumference 85 cm or greater are at increased risk of comorbidity. The presence of the metabolic syndrome has been demonstrated to increase the likelihood of histologically confirmed NASH by 40% and is independently associated with increased overall mortality among patients with NAFLD [8, 9]. Lifestyle and diet play an important role in obesity. The consumption of a high‐calorie diet with elevated amounts of fructose, refined carbohydrates, and saturated fats, coupled with a sedentary lifestyle, has been associated with higher rates of NAFLD. Given the role of insulin resistance in the pathogenesis of NAFLD and metabolic syndrome, it is not surprising that T2DM is a risk factor for the development and progression of NAFLD, and insulin resistance is almost universal in patients with NASH. In regard to the other constituents of metabolic syndrome, hypertension and dyslipidemia (hypertriglyceridemia and low high‐density lipoprotein in particular) are associated with an increased risk of NAFLD [8, 10]. Age and sex also appear to be risk factors for NAFLD. The prevalence of NAFLD and stage of liver disease increases with age. Epidemiologic studies have demonstrated a higher prevalence of NAFLD in men than women, and advanced disease tends to present later in life in women, suggesting a potentially protective role for sex hormones. As noted above, there is a variability in the prevalence of NAFLD based on ethnicity/geographic region (i.e. Asia Pacific, Latin America, and the Middle East) and these differences are thought to be related to the frequency of specific gene alleles, such as PNPLA3. Alcohol‐related liver disease is another common cause of hepatic steatosis, but it is a separate diagnosis and is not discussed here. However, NAFLD and alcohol‐related liver disease are not mutually exclusive and are often present concurrently. Other conditions that have been associated with NAFLD are hypothyroidism, obstructive sleep apnea, polycystic ovarian syndrome, vitamin D deficiency, hypogonadism, hypopituitarism, osteoporosis, and psoriasis [8]. Patients with NAFLD have an increased overall mortality compared with patients without NAFLD. Cardiovascular disease is the primary cause of death in patients with NAFLD without advanced fibrosis, followed by cancer. Liver‐specific mortality increases with progressive fibrosis and is highest in those with NASH and advanced fibrosis [6]. The extent of underlying hepatic fibrosis, not steatosis, is the most important predictor of prognosis and liver‐related morbidity and mortality in patients with NAFLD. As noted above, NASH is typically associated with progression of liver fibrosis. Patients with NAFLD and no fibrosis at baseline generally progress to stage 1 fibrosis over approximately 14 years, whereas patients with NASH and no fibrosis at baseline progress to stage 1 fibrosis over around 7 years. Previous data suggested that around 20% of patients with NAFLD also have NASH; however, recent studies suggest that this may be an underestimate [11]. Prospective studies have revealed progression from simple steatosis to NASH in 23–44% of patients over a period of 2.2–6.6 years [12]. Other evidence suggests that NASH is a dynamic process with fluctuating progression and resolution [11]. It is estimated that approximately 25–40% of patients with NASH will develop progressive liver fibrosis and that 11% of patients with NASH will progress to cirrhosis [13]. The typical progression of NAFLD is illustrated in Figure 17.1. Figure 17.1 Prevalence and progression of non‐alcoholic fatty liver disease. NAFLD, non‐alcoholic fatty liver disease; NASH, non‐alcoholic steatohepatitis. NAFLD, including lean NAFLD, is an independent risk factor for cardiovascular disease. Studies have demonstrated that patients with NAFLD have an approximately 65% increased risk of cardiovascular disease and are more likely to die from cardiovascular disease than liver disease in those without advanced fibrosis [14]. Thus, counseling on risk factors for cardiovascular disease, including smoking and metabolic disease, is essential to the general care of patients with NAFLD. Obesity is commonly associated with an increased risk of malignancy. Recent evidence suggests that NAFLD may potentiate the risk of cancer in patients with obesity, and may also be an independent risk factor. Hepatocellular carcinoma (HCC) is the most common malignancy in patients with NAFLD, but increased rates of uterine, gastric, pancreatic, and colonic cancer have also been observed [15]
17
Non‐Alcoholic Fatty Liver Disease
Introduction
Definition
Epidemiology
Pathophysiology
Risk Factors
Natural History/Prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








