Gastrointestinal endoscopy is a rapidly evolving field. Techniques in endoscopy continue to become more sophisticated, as do the devices and platforms, particularly in colonoscopy and endoscopic resection. This article reviews new platforms for endoscopic imaging of the colon, and discusses new endoscopic accessories and developments in endoscopic resection.
Key points
- •
The Endotics System, Aer-O-Scope and Colon Capsule Endoscopy are potential alternative platforms for screening colonoscopy but lack therapeutic capabilities.
- •
The Invendoscope has therapeutic capabilities but further improvements are needed for reliable performance.
- •
Neoguide has potential for accurately identifying scope position which can assist identifying location of lesions.
- •
Responsive insertion technology is promising new endoscope technology which can be advantageous in patients with failed colonoscopy as a result of sharp angulations (post pelvic surgery) or a narrowed lumen.
- •
Colon capsule endoscopy (CCE) offers a minimally invasive and painless method of imaging the colon without the need for sedation, however optimal bowel preparation is essential. CCE is a feasible and safe tool for visualization of colonic mucosa in patients with incomplete colonoscopy attributable to difficult anatomy, however it remains a diagnostic tool without therapeutic capabilities.
- •
Advanced polypectomy may be facilitated by new injection solutions.
- •
Improved through-the-scope clips and over-the-scope clips are available to manage post polypectomy bleeding and perforations.
Gastrointestinal endoscopy is a rapidly evolving field. Techniques in endoscopy continue to become more sophisticated, as do the devices and platforms, particularly in colonoscopy and endoscopic resection. This article reviews new platforms for endoscopic imaging of the colon, and discusses new endoscopic accessories and developments in endoscopic resection.
New platforms for endoscopic imaging of the colon
Colonoscopy is the preferred screening modality for colorectal cancer, and cecal intubation rates of greater than 95% are expected. However, colonoscopy can be technically difficult, owing to redundant colon or because of angulated or fixed sigmoid colon related to pelvic surgery or diverticular disease. An inability to examine the entire colon undermines the benefits of screening, especially in the proximal colon.
Based on the need for better performance of screening methods and better acceptance by patients, the following devices and technology have been developed to assist navigation through the colon, some of which are equipped with therapeutic capabilities ( Box 1 ).
| Diagnostic Platforms | Therapeutic Platforms |
|---|---|
| Endotics System | Invendoscope |
| Aer-O-Scope | NeoGuide |
| Colon Capsule Endoscope |
New platforms for endoscopic imaging of the colon
Colonoscopy is the preferred screening modality for colorectal cancer, and cecal intubation rates of greater than 95% are expected. However, colonoscopy can be technically difficult, owing to redundant colon or because of angulated or fixed sigmoid colon related to pelvic surgery or diverticular disease. An inability to examine the entire colon undermines the benefits of screening, especially in the proximal colon.
Based on the need for better performance of screening methods and better acceptance by patients, the following devices and technology have been developed to assist navigation through the colon, some of which are equipped with therapeutic capabilities ( Box 1 ).
| Diagnostic Platforms | Therapeutic Platforms |
|---|---|
| Endotics System | Invendoscope |
| Aer-O-Scope | NeoGuide |
| Colon Capsule Endoscope |
Diagnostic platforms
Navigation through a tortuous and angulated colon can be challenging for the endoscopist. Several platforms have been devised to assist navigation by minimizing looping or by taking advantage of peristalsis to propel the device.
Endotics System
The Endotics System (ES) (Era Endoscopy, Pisa, Italy) is a disposable robotic probe with a head, steerable tip, flexible body (17 mm diameter), and tail (7.5 mm diameter, 180 cm length), connected to a control box with an electropneumatic connector. A camera, light-emitting diode light source, and channels for air and water are incorporated into the head of the device and can be steered 180° in all directions. Two vacuum anchors located in the proximal and distal ends of the device move sequentially to provide an “inchworm-like” movement of the apparatus, thereby negotiating complex contours of the colon as the probe advances through the lumen ( Fig. 1 ).

Tumino and colleagues performed both standard colonoscopy and ES colonoscopy in 71 patients. Cecal intubation rate was lower in the ES group (81.6% vs 94.3%, P = .03). Similarly, mean procedural time was longer in the ES group (45.1 vs 23.7 minutes, P <.0001); however, polyp detection rates were higher when compared with standard colonoscopy (sensitivity 93.3%, specificity 100%, positive predictive value 100%, negative predictive value 97.7%). None of patients in the ES group required sedation, an advantage attributable to the inchworm-like motion of the endoscope, exerting minimal forces on the colonic wall and thereby preventing pain caused by stretching of the mesocolon.
Therefore, ES may allow performance of diagnostic colonoscopy without the need for sedation while providing a high diagnostic yield. However, it is limited by lower cecal intubation rate, lengthier procedural times, and lack of a therapeutic channel. Further improvement of the device, in addition to larger studies, are needed before incorporation into clinical practice can be considered.
Aer-O-Scope
The Aer-O-Scope (GI View, Ramat Gan, Israel) is a self-propelling and self-navigating diagnostic robotic colonoscope, comprising a workstation and disposable unit ( Fig. 2 ). The disposable unit consists of a rectal introducer, supply cable, and endoscope embedded within a mobile balloon. The rectal introducer is a hollow tube with a balloon attached to its outer surface, which is inflated and fixed in the rectum. The endoscope is passed through the introducer. The rectal balloon provides a seal, preventing gas leakage. Carbon dioxide is insufflated between both balloons, allowing forward propulsion of the mobile balloon (which is attached to the endoscope).
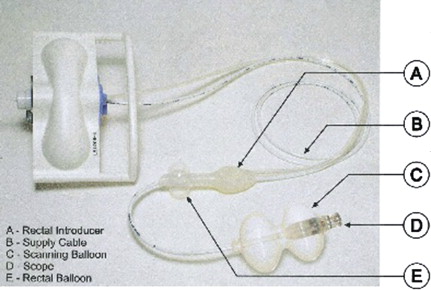
The user interface is provided by a personal computer–based workstation operated by a control box, which is brought to the bedside. The workstation is connected to the scope by the disposable supply cable, and controls the gas pressure behind and within the mobile balloon. The pressures in front of, inside of, and behind the balloon are measured by sensors and automatically adjusted by a computerized algorithm. The operator can choose the operation mode (forward, backward, pause, and stop). Data from the digital camera are displayed on a screen and recorded on a compact disc. The operator can withdraw the instrument by gently pulling on the supply cable. Once the procedure is completed, the colon is deflated, the rectal seal is released, and the device is removed.
Initial proof-of-concept studies were performed in animal models followed by clinical experiments. Vucelic and colleagues studied the Aer-O-Scope in 12 healthy volunteers (age 20–43 years). The cecum was reached in 10 subjects. The instrument could not be passed beyond the hepatic flexure in the remaining 2 cases. Mean cecal intubation time was 14 minutes. Mild sweating and bloating was experienced by 40% of subjects. Subsequently, all subjects underwent follow-up colonoscopy, and 40% were found to have mild submucosal petechial lesions, likely a result of friction between the device and colonic mucosa.
The major limitation of this device is the lack of therapeutic capabilities. However, the device is easy to use, requires minimal training, and may have utility in helping to meet the increasing demand for screening colonoscopy.
Colon Capsule Endoscopy
Small-bowel endoscopy was revolutionized with the advent of wireless capsule endoscopy, first reported more than a decade ago. Applications of the device have broadened as newer generations of the capsule continue to improve. The first generation of colon capsule endoscopy (CCE) was developed in 2006. CCE offers a minimally invasive and painless method of imaging the colon without the need for sedation or gas insufflation.
Adequate colon preparation is essential for successful CCE, for several reasons. Bowel cleansing improves mucosal visualization, facilitates propulsion of the capsule through the small bowel to reach the colon, and results in filling the colonic lumen with clear liquids, which improves mucosal visualization and diminishes air bubbles. The recommended bowel preparation regimen was recently modified to optimize colonic examination. Patients commence a clear liquid diet the day before the procedure. Standard bowel preparation regimens should be administered, preferably in a split-dosage fashion. Booster preparations are necessary to help propel the capsule and to complete visualization of the colonic mucosa. The progression of the capsule is monitored with a real-time viewing system performed with Rapid Access Real Time Tablet DC (Given Imaging, Yoqneum, Israel). A prokinetic is recommended if the capsule is retained in the stomach for longer than 1 hour. Contraindications to small bowel capsule endoscopy apply to CCE ( Box 2 ).
Dysphagia or swallowing disorder
Prior major abdominal surgery of the gastrointestinal tract
Known or suspected bowel obstruction
Presence of cardiac pacemaker
Other implanted electromedical device
Pregnancy
The first-generation colon capsule PillCam (Given Imaging) measured 31 × 11 mm and was equipped with dual cameras and implemented optics, with a total operating time of approximately 10 hours. The angle of view from each imager was 156°. The capsule entered a “sleep” mode for the first 1 hour 45 minutes, conserving battery power for colonic examination as the capsule traversed the small bowel. Subsequently, the capsule automatically reactivated and started transmission of distal ileal images before exploring the colon. The second-generation PillCam colon capsule (PCC-2; Given Imaging) is slightly larger, measuring 31.5 × 11.6 mm. The angle of view from both imagers is widened to 172°, allowing for almost 360° coverage of the colon. In addition, the capsule is equipped with an adaptable image acquisition rate, which adjusts to the speed of capsule propulsion through the intestine, helping to conserve battery energy and optimize video length. It first begins capturing at a low rate of 14 images per minute until small-bowel images are detected. The capsule also has the ability to “adapt” its frame rate. When stationary, PCC-2 captures 4 frames per second, and increases to 35 frames per second while moving. The data recorder (DR3) directs the medical staff and the patient through the procedure with vibration and noise alerts on its liquid-crystal diode screen to alert the patient to continue the preparation according to the protocol. The software (RAPID) contains additional diagnostic features for video processing and viewing.
Two meta-analyses of patients undergoing CCE have been published to date, including 9 studies examining the first-generation colon capsule. The average sensitivity and specificity for detection of significant findings (polyps ≥6 mm size or ≥3 polyps irrespective of size) was 68% to 69% and 82% to 86%, respectively.
Two studies evaluated the second-generation capsule, with improved sensitivity for significant findings (84%–89%). The specificity varied from 64% for polyps 6 mm or larger in size to 95% for polyps 10 mm or larger. The low specificity resulted from polyps detected on capsule endoscopy but not seen at colonoscopy.
CCE was associated with no major complications in more than 1500 procedures. In addition, CCE has a very low rate of technical failures. Capsule excretion rate of about 90% is likely underestimated, as this was measured 8 hours after ingestion in most studies.
Sung and colleagues recently performed a multicenter prospective cohort study to investigate the accuracy of CCE for assessing the activity of colonic inflammation, using optical colonoscopy as the gold standard in 100 patients. Patients at higher risk for capsule retention (eg, Crohn disease, small-bowel tumors, radiation enteropathy, and previous intestinal surgery) were excluded. Most capsules were excreted naturally, with few (n = 9) being retrieved at colonoscopy. This study demonstrated high sensitivity for detecting mucosal inflammation (89%) but low specificity and negative predictive values of 75% and 65%, respectively. In other words, CCE failed to detect active disease in 25% of cases. The most common causes of failure to detect mucosal abnormalities were suboptimal bowel preparation, luminal bubbles, and quick colonic transit.
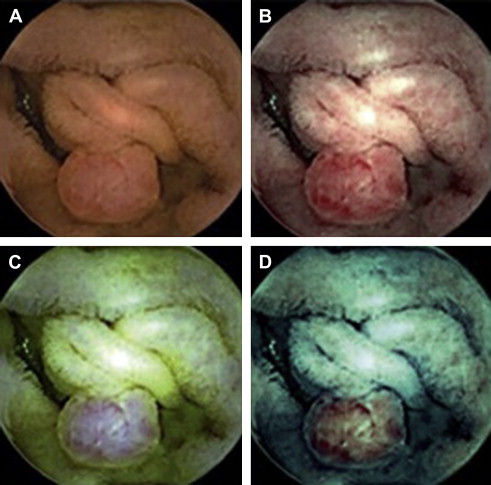
Recently, chromoendoscopy was incorporated into the capsule endoscopy system. Fujinon intelligent color enhancement (FICE) takes an ordinary endoscopic image and arithmetically processes the reflected photons to reconstitute virtual images for selected wavelengths. In a feasibility study by Pohl and colleagues, 10 patients underwent small-bowel capsule endoscopy for evaluation of obscure gastrointestinal bleeding. Higher-quality images of polyps and angiodysplasias were obtained ( Fig. 3 ). If this technology were incorporated into the CCE, perhaps further improvement in diagnostic yield would be possible.
CCE is not yet endorsed as a screening method for colon cancer. The first-generation CCE was associated with low sensitivity for polyps, but this has improved considerably with the second-generation capsule. This advance is partly due to improvements in the technology and alterations in the bowel preparation regimen. Some patients may prefer CCE over conventional colonoscopy given the lack of sedation, its noninvasive nature, and oral (rather than rectal) route of insertion. CCE is also a feasible and safe tool for visualization of the colonic mucosa in patients with incomplete colonoscopy attributable to difficult anatomy. However, CCE remains a diagnostic tool without therapeutic capabilities, and its performance depends on an optimal bowel preparation.
Therapeutic platforms
Therapeutic interventions are required in up to 30% of screening colonoscopies, ranging from minor polypectomy to advanced mucosal resections. Therapeutic platforms must provide an adequate working channel compatible with accessories that facilitate endoscopic resection. The following platforms provide an alternative to current standard colonoscopy, and have therapeutic capabilities.
Invendoscope
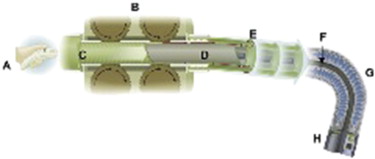
The Invendo SC20 (Invendo Medical, Kissing, Germany) uses principles of colonoscopy attached to a motor-driven device ( Figs. 4 and 5 ). The design of this disposable device is similar to that of conventional endoscopes, allowing for insufflation, rinsing, and suction with a 3.1-mm working channel. Insertion and withdrawal of a colonoscope is replaced by an “inverted-sleeve” mechanism while a hand-held control unit activates all the endoscopic and software functions. The wheels grip onto the inner side of the inverted sleeve, causing the sleeve and inner sheath to drive either forward or backward. If the ‘‘forward’’ or ‘‘backward’’ key is not pressed by the operator, the wheels in the endoscope driving unit automatically stop. The endoscope tip can be flexed electrohydraulically 180° in any direction by moving a joystick on the hand-held device. The latest version of the Invendo SC20 is CE marked (Conformité Européenne).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






