Neuropathic Bladder Disorders: Introduction
The urinary bladder is a visceral smooth-muscle organ but is under voluntary control from the cerebral cortex. Normal bladder function requires coordinated interaction of afferent and efferent components of both the somatic and autonomic nervous systems. Because many levels of the nervous system are involved in the regulation of voiding function, neurologic diseases often cause changes in bladder function. Examples are multiple sclerosis, spinal cord injury, cerebrovascular disease, Parkinson disease, diabetes mellitus, meningomyelocele, and amyotrophic lateral sclerosis. Injury to the sacral roots or pelvic plexus from spinal surgery, herniation of an intervertebral disk, or pelvic surgery (hysterectomy, abdominoperineal resection) can also cause neuropathic bladder.
Significant bladder dysfunction may occur as a result of poor voiding habits in childhood or of degenerative changes in bladder muscle and nerve endings caused by aging, inflammation, or anxiety disorders. All the above conditions can disrupt efficient reflex coordination between sphincter and bladder, and with time, this leads to symptomatic dysfunction.
Normal Vesical Function
The bladder wall is composed of a syncytium of smooth-muscle fibers that run in various directions; however, near the internal meatus, three layers are distinguishable: a middle circular layer and inner and outer longitudinal layers. In females, the outer layer extends down the entire length of the urethra, while in males, it ends at the apex of the prostate. The muscle fibers become circular and spirally oriented around the bladder–urethra junction. The middle circular layer ends at the internal meatus of the bladder and is most developed anteriorly. The inner layer remains longitudinally oriented and reaches the distal end of the urethra in females and the apex of the prostate in males. The convergence of these muscle fibers forms a thickened bladder neck, which functions as the internal smooth-muscle sphincter.
The normal bladder is able to distend gradually to a capacity of 400–500 mL without appreciable increase in intravesical pressure. When the sensation of fullness is transmitted to the sacral cord, the motor arc of the reflex causes a powerful and sustained detrusor contraction and urination if voluntary control is lacking (as in infants). As myelinization of the central nervous system progresses, the young child is able to suppress the sacral reflex so that he or she can urinate when it is appropriate.
The functional features of the bladder include (1) a normal capacity of 400–500 mL, (2) a sensation of fullness, (3) the ability to accommodate various volumes without a change in intraluminal pressure, (4) the ability to initiate and sustain a contraction until the bladder is empty, and (5) voluntary initiation or inhibition of voiding despite the involuntary nature of the organ.
In both males and females, there are two sphincteric elements: (1) an internal involuntary smooth-muscle sphincter at the bladder neck and (2) an external voluntary striated muscle sphincter from the prostate to the membranous urethra in males and at the midurethra in females.
The bladder neck sphincter is a condensation of smooth muscle of the detrusor. This area is rich in sympathetic innervation. Active contraction of the bladder neck region occurs simultaneously with seminal emission, just before ejaculation. In the filling phase, the bladder neck remains closed to provide continence. It opens during both spontaneous contraction and contraction induced by stimulation of the pelvic nerve.
The external sphincter is composed of slow-twitch, small striated muscle fibers in addition to longitudinal and circularly oriented smooth-muscle fibers. This sphincter maintains a constant tonus and is the primary continence mechanism. While the resting tone is maintained by the slow-twitch striated muscle and the smooth muscles, it can be voluntarily increased by contraction of the striated muscles of the pelvic floor (eg, levator ani), which contain both fast- and slow-twitch fibers.
Relaxation of the sphincter is mostly a voluntary act without which voiding is normally inhibited. Nitric oxide released by parasympathetic nerve endings has been proposed to be the neurotransmitter for sphincter relaxation. Failure to initiate sphincteric relaxation is a mechanism of urinary retention often seen in children with dyssynergic voiding. In infancy, the detrusor behaves in an uninhibited fashion. As the central nervous system matures, children learn to suppress or enhance the micturition reflex through voluntary contraction or relaxation of the pelvic musculature.
The function of the ureterovesical junction is to prevent backflow of urine from the bladder to the upper urinary tract. Longitudinal muscle from the ureter contributes to the makeup of the trigone. Stretching of the trigone has an occlusive effect on the ureteral openings. During normal detrusor contraction, the increased pull on the ureters prevents reflux of urine. Conversely, the combination of detrusor hypertrophy and trigonal stretch owing to residual urine can significantly obstruct the flow of urine from the ureters into the bladder.
The lower urinary tract receives afferent and efferent innervation from both the autonomic and somatic nervous systems. The parasympathetic innervation originates in the second to fourth sacral segments. The cholinergic postganglionic fibers supply both the bladder and smooth-muscle sphincter. The sympathetic nerves originate at T10–L2. The noradrenergic postganglionic fibers innervate the smooth muscles of the bladder base, internal sphincter, and proximal urethra. Somatic motor innervation originates in S2–4 and travels to the striated urethral sphincter via the pudendal nerve. Some motor neurons to the tonic small muscle fibers of the striated sphincter may also project through the pelvic nerve (Crowe et al, 1989).
There are both somatic and visceral afferents from the bladder and urethra. The somatic afferent is carried by the pudendal nerve, while the visceral afferent projects through the sympathetic and parasympathetic nerves to their respective spinal areas.
The normal afferent pathway is mediated largely by Aδ-fibers, which send information about the state of bladder fullness to the pontine micturition center (PMC). C-fibers respond to chemical irritation or cold. After spinal injury, C-fibers become more prominent contributing to neurogenic detrusor overactivity (NDO). The common sources of afferent information for either pathway are likely to be afferents from the urothelium, lamina propria, and afferents that originate in the bladder wall (Birder et al, 2010; Clemens, 2010; de Groat and Yoshimura, 2010). On the other hand, the thoracolumbar visceral afferents may transmit discomfort and pain.
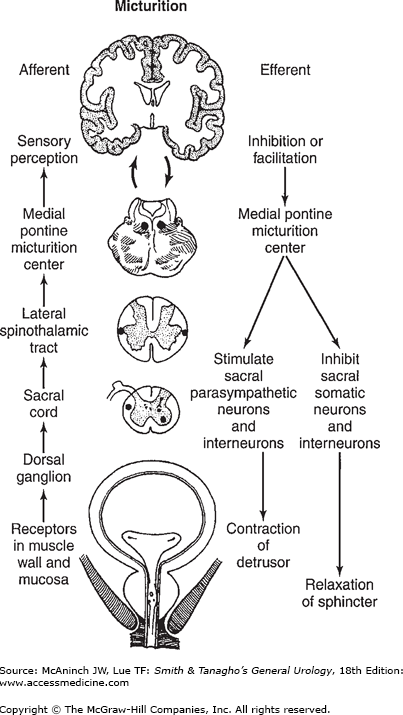
Intact reflex pathways via the spinal cord and the pons are required for normal micturition. Afferents from the bladder are essential for the activation of the sacral center, which then causes detrusor contraction, bladder neck opening, and sphincteric relaxation. The periaqueductal gray (PAG) in the rostral brain stem is the integration center for signals from bladder, cerebral cortex, and hypothalamus. The PMC, through its connection with the sacral center, may send either excitatory or inhibitory impulses to regulate the micturition reflex (Tai et al, 2009). Electrical or chemical stimulation of the neurons in the medial PMC generates contraction of the detrusor and relaxation of the external sphincter. Disruption of pontine control, as in upper spinal cord injury, leads to contraction of the detrusor without sphincteric relaxation (detrusor–sphincter dyssynergia).
In pathologic conditions affecting the urethra (eg, urethritis or prostatitis) or the bladder (eg, cystitis or obstructive hypertrophy), overactive detrusor activity may occur because of facilitation of the micturition reflex (Figure 28–1).
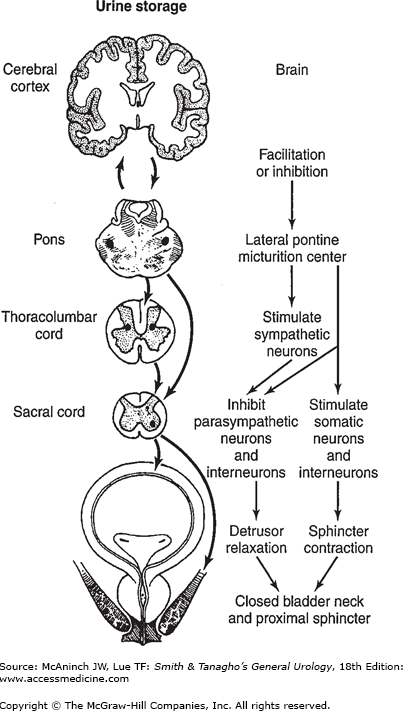
The external sphincter plays an important role in urine storage. The afferent signals from pelvic and pudendal nerves activate both the sacral and lateral PMC; this enhances sphincteric contraction while suppressing the parasympathetic impulse to the detrusor. Voluntary tightening of the sphincter can also inhibit the urge to urinate. In addition, activation of sympathetic nerves increases urethral resistance and facilitates bladder storage (Figure 28–2).
Although micturition and urine storage are primary functions of the autonomic nervous system, these are under voluntary control from suprapontine cerebral centers, so that other groups of muscles (arm, leg, hand, bulbocavernosus) can be integrated to assist in urination at the appropriate time and place. Cerebral lesions (eg, from tumor, Parkinson’s disease, vascular accident) are known to affect the perception of bladder sensation and result in voiding dysfunction.
In parasympathetic innervation, acetylcholine and nicotinic receptors mediate pre- to postganglionic transmission, while acetylcholine and M3 muscarinic receptors mediate the postganglionic neuron–smooth muscle transmission. In some species, adenosine triphosphate (ATP) is released with acetylcholine and acts on purinoceptors (P2) in the smooth-muscle cell. In sympathetic nerves, noradrenaline can act on the beta-3 adrenoreceptors to relax the detrusor or the alpha-1-receptors to contract the bladder neck and the external sphincter (Birder et al, 2010).
In addition, many neuropeptides, which usually colocalize with the classic transmitters, are also found in the genitourinary tract. Neuropeptide Y, enkephalin, and vasoactive intestinal polypeptide (VIP) are found in cholinergic postganglionic neurons, while calcitonin gene-related peptide (CGRP), VIP, substance P, cholecystokinin, and enkephalins are distributed in sacral visceral afferent fibers. These peptides are thought to be involved in modulation of efferent and afferent neurotransmissions.
Please refer to Chapter 27 (“Neurophysiology and Pharmacology of the Lower Urinary Tract”) for more detail discussion of the neurophysiology.
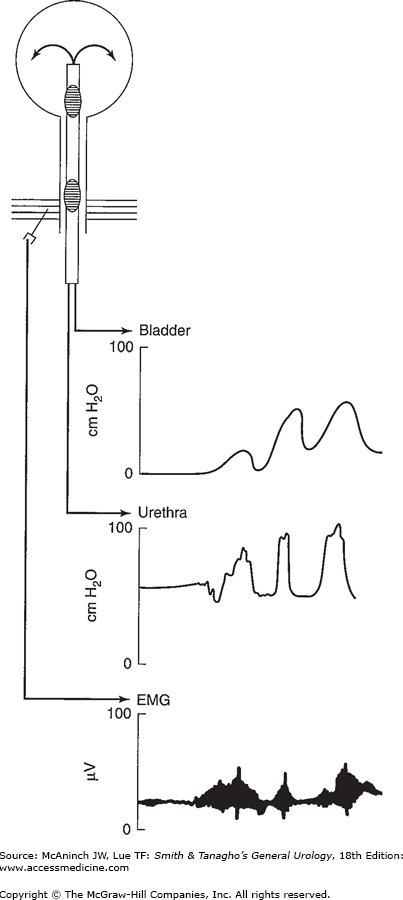
Urodynamic studies are techniques used to obtain graphic recordings of activity in the urinary bladder, urethral sphincter, and pelvic musculature. The current method involves use of water to transfer pressure to a transducer housed near a polygraph or use of a transducer-tipped catheter to transfer pressure recordings directly to a polygraph. Pressure recordings can be complemented by electromyography of the perineal musculature, ultrasound, or radiography (Figure 28–3).
Figure 28–3.
Simultaneous recording of bladder and urethral pressure as well as electromyographic recording of the external sphincter. Note the dyssynergic response. With bladder contraction, there is increased activity in the external sphincter and pelvic floor, as recorded by the intraurethral pressure and electromyogram tracings.
Uroflowmetry is the study of the flow of urine from the urethra. Uroflowmetry is best performed separately from all other tests and, whenever possible, as a standard office screening or monitoring procedure. The normal peak flow rate for males is 20–25 mL/s and for females 20–30 mL/s. Lower flow rates suggest outlet obstruction or a weak detrusor; higher flow rates suggest bladder spasticity or excessive use of abdominal muscles to assist voiding. Intermittent flow patterns generally reflect spasticity of the sphincter or straining to overcome resistance in the urethra or the prostate.
Cystometry is the urodynamic evaluation of the reservoir function of the bladder. Cystometry is most informative when combined with studies of the external urethral sphincter and pelvic floor.
Normal bladder capacity is 400–500 mL. Bladder pressure during filling should remain low up to the point of voiding. The first desire to void is generally felt when the volume reaches 150–250 mL, but detrusor-filling pressure should remain unchanged until there is a definite sense of fullness at 350–450 mL, the true capacity of the bladder. Detrusor contractions before this point are considered abnormal. Normal voiding pressures in the bladder should not rise above 30 cm of water pressure. With normal voiding, there should be no residual urine, and voiding should be accomplished without straining.
Normal voiding requires a synergic action of the bladder (contraction) and urethra (relaxation). High pressures in the bladder during voiding reflect abnormal resistance in the urethral outlet. Increased outlet resistance can result from prostatic enlargement, urethral stricture, bladder neck contracture, or spasm of the external urethral sphincter. Low resistance in the urethral outlet generally reflects compromised function of the sphincter mechanism. Recording of urethral pressures with the bladder at rest as well as during contraction helps determine the presence of functional or anatomic disorders.
With electromyography, the activity of the striated urethral muscles can be monitored without obstructing the urethral lumen. In the normal urethra, activity increases slightly as the bladder fills and falls precipitously just before voiding begins. Denervation results in an overall decrease in activity as well as production of denervation potentials. An overall increase in activity reflects a state of hyperreflexia. The technique provides a sensitive assessment of urethral and pelvic muscle behavior. The disadvantages of the technique are dependence on accurate needle position and a tendency to record artifacts.
Abnormal Vesical Function
The traditional classification was according to neurologic deficit. Thus, the terms motor, spastic, upper motor neuron, reflexic, and uninhibited were used to describe dysfunction found with injury above the spinal cord micturition center. The classification system recommended by the standardization committee for the International Continence Society includes description of bladder activity (normal, overactive, areflexic, impaired), bladder compliance (normal, decreased, increased), smooth sphincter activity (synergic, dyssynergic), striated sphincter activity (synergic, dyssynergic, fixed tone), and sensation (normal, absent, impaired) (Abrams et al, 2002).
Descriptions of neuromuscular dysfunction of the lower urinary tract should be individualized because no two neural injuries (no matter how similar) result in the same type of dysfunction.
Most lesions above the level of the cord where the micturition center is located will cause bladder overactivity. Sacral reflex arcs remain intact, but loss of inhibition from higher centers results in overactive bladder and sphincter behavior on the segmental level. The degree of spasticity varies between the bladder and sphincter, from lesion to lesion, and from patient to patient with similar lesions.
Common lesions found above the brain stem that affect voiding include dementia, vascular accidents, multiple sclerosis, tumors, and inflammatory disorders such as encephalitis or meningitis. These lesions can produce a wide range of functional changes, including precipitate urge, frequency, residual urine, retention of urine, recurrent urinary tract infections, and gross incontinence. Symptoms range from mild to disabling. Obviously, incontinence is especially troublesome. If the lesion is above the PMC, detrusor–striated sphincter dyssynergia usually does not occur. However, leakage may occur because the need to void cannot be felt or because the sphincter becomes more relaxed and can no longer inhibit spontaneous voiding. Lesions of the internal capsule include vascular accidents and Parkinson’s disease. Both spastic and semiflaccid voiding disorders are found with these lesions.
Spinal cord injury can be the result of trauma, herniated intervertebral disk, vascular lesions, multiple sclerosis, tumor, syringomyelia, or myelitis, or it may be iatrogenic. Traumatic spinal cord lesions are of greatest clinical concern. Partial or complete injuries may cause equally severe genitourinary dysfunction. Sphincter spasticity and voiding dyssynergia can lead to detrusor hypertrophy, high voiding pressures, ureteral reflux, or ureteral obstruction. With time, renal function may be compromised. If infection is combined with back pressure on the kidney, loss of renal function can be particularly rapid.
Spinal cord injuries at the high thoracic (above T6) and cervical levels are often associated with (1) detrusor–sphincter (both smooth and striated) dyssynergia and (2) autonomic dysreflexia. Because the lesions occur above the sympathetic outflow from the cord, hypertensive blood pressure fluctuations, bradycardia, and sweating can be triggered by insertion of a catheter, mild overdistention of the bladder with filling, or dyssynergic voiding (see later). When a complete lesion is near the levels of sympathetic outflow, patient may develop bladder overactivity with no sensation, smooth sphincter synergy but striated sphincter dyssynergia.
In summary, the overactive neuropathic bladder is typified by (1) reduced capacity, (2) involuntary detrusor contractions, (3) high intravesical voiding pressures, (4) marked hypertrophy of the bladder wall, (5) spasticity of the pelvic-striated muscle, and (6) autonomic dysreflexia in cervical cord lesions.
The most common cause of flaccid neuropathic bladder is injury to the spinal cord at the micturition center, S2–4. Other causes of anterior horn cell damage include infection due to poliovirus or herpes zoster and iatrogenic factors such as radiation or surgery. Herniated disks can injure the micturition center but more commonly affect the cauda equina or sacral nerve roots. Myelodysplasias could also be grouped here, but the mechanism is actually failure in the development or organization of the anterior horn cells. Lesions in this region of the cord are often incomplete, with the result commonly being a mixture of spastic behavior with weakened muscle contractility. Mild trabeculation of the bladder may occur. External sphincter and perineal muscle tone are diminished. Urinary incontinence usually does not occur in these cases because of the compensatory increase in bladder storage. The bladder pressure is low and little outlet resistance is needed to provide continence. Evacuation of the bladder may be accomplished by straining, but with variable success.
Flaccid neuropathic bladder also results from a variety of neuropathies, including diabetes mellitus, tabes dorsalis, pernicious anemia, and posterior spinal cord lesions. Here, the mechanism is not injury of the detrusor motor nucleus but a loss of sensory input to the detrusor nucleus or a change in motor behavior due to loss of neurotransmission in the dorsal horns of the cord. The end result is the same. Loss of perception of bladder filling permits overstretching of the detrusor. Atony of the detrusor results in weak, inefficient contractility. Capacity is increased and residual urine significant.
In summary, the flaccid neuropathic bladder is typified by (1) large capacity, (2) lack of voluntary detrusor contractions, (3) low intravesical pressure, (4) mild trabeculation (hypertrophy) of the bladder wall, and (5) decreased tone of the external sphincter.
Another cause of atonic neuropathic bladder is peripheral nerve injury. This category includes injury caused by radical surgical procedures such as low anterior resection of the colon or radical hysterectomy. Impaired innervation of detrusor and both the smooth and striated sphincters create outflow resistance and incomplete voiding. The end result is a bladder that stores poorly owing to failure to accommodate with filling.
Radiation therapy can result in denervation of the detrusor or sphincters. More commonly, it damages the detrusor, resulting in fibrosis and loss of distensibility. Other inflammatory causes of injury to the detrusor include chronic infection, interstitial cystitis, and carcinoma in situ. These lesions produce a fibrotic bladder wall with poor distensibility.
Pelvic fracture often damages the nerves to the external sphincter. Selective denervation of the external sphincter muscle, with incontinence, can follow if the bladder neck is not sufficiently competent. Radical surgery in the perineum may affect sensory but not motor innervation of the external sphincter.
Immediately following severe injury to the spinal cord or conus medullaris, regardless of level, there is a stage of flaccid paralysis, with numbness below the level of the injury. The smooth muscle of the detrusor and rectum is affected. The result is detrusor overfilling to the point of overflow incontinence and rectal impaction.
Spinal shock may last from a few weeks to 6 months (usually 2–3 months). Reflex response in striated muscle is usually present from the time of injury but is suppressed. With time, the reflex excitability of striated muscle progresses until a spastic state is achieved. Smooth muscle is much slower to develop this hyperreflexic activity. Therefore, urinary retention is the rule in the early months following injury.
Urodynamic studies are indicated periodically to monitor the progressive return of reflex behavior. In the early recovery stages, a few weak contractions of the bladder may be found. Later, in injuries above the micturition center, more significant reflex activity will be found. Low-pressure storage can be managed via intermittent catheterization. High-pressure storage should be addressed early to avoid problems with the upper urinary tract.
A seldom used but valuable test is instillation of ice water. A strong detrusor contraction in response to filling with cold saline (3.3°C [38°F]) is one of the first indications of return of detrusor reflex activity. This test is of value in differentiating upper from lower motor neuron lesions early in the recovery phase.
Activity of the bladder after the spinal shock phase depends on the site of injury and extent of the neural lesion. With upper motor neuron (suprasegmental) lesions, there is obvious evidence of spasticity toward the end of the spinal shock phase (eg, spontaneous spasms in the extremities, spontaneous leakage of urine or stool, and, possibly, the return of some sensation). A plan of management can be made at this time. A few patients will retain the ability to empty the bladder reflexively by using trigger techniques, that is, by tapping or scratching the skin above the pubis or external genitalia. More often, detrusor overactivity must be suppressed by anticholinergic medication to prevent incontinence. Evacuation of urine can then be accomplished by intermittent catheterization. Although incomplete lesions are more amenable to this approach than complete lesions, 70% of complete lesions ultimately can be managed using this program. Patients who cannot be managed in this way can be evaluated for sphincterotomy, dorsal rhizotomy, diversion, augmentation, or a bladder pacemaker procedure.
In cases of lower motor neuron lesions, it is difficult to distinguish spinal shock from the end result of the injury. Spontaneous detrusor activity cannot be elicited on urodynamic evaluation. If the bladder is allowed to fill, overflow incontinence will occur. Striated muscle reflexes will be suppressed or absent. The bladder may be partially emptied by the Credé maneuver (ie, by manually pushing on the abdomen above the pubic symphysis) or, preferably, by intermittent catheterization.
The diagnosis of a neuropathic bladder disorder depends on a complete history and physical (including neurologic) examination, as well as use of radiologic studies (voiding cystourethrography, excretory urography, computed tomography scanning, magnetic resonance imaging, when necessary); urologic studies (cystoscopy, ultrasound); urodynamic studies (cystometry, urethral pressure recordings, uroflowmetry); and neurologic studies (electromyography, evoked potentials). Patients should be reevaluated often as recovery progresses.
Overactive bladder results from extensive neural damage above sacral cord but below the PMC. The bladder functions on the level of spinal segmental reflexes, without efficient regulation from higher brain centers.
The severity of symptoms depends on the site and extent of the lesion as well as the length of time from injury. Symptoms include involuntary urination, which is often frequent, spontaneous, scant, and triggered by spasms in the lower extremities. A true sensation of fullness is lacking, although vague lower abdominal sensations due to stretch of the overlying peritoneum may be felt. The major nonurologic symptoms are those of spastic paralysis and objective sensory deficits.
A complete neurologic examination is most important. The sensory level of the injury needs to be established, followed by assessment of the anal, bulbocavernosus, knee, ankle, and toe reflexes. These reflexes vary in degree of hyperreflexia on a scale of 1–4. Levator muscle tone and anal tone should be gauged separately, also on a scale of 1–4. Bladder volumes in established lesions are usually <300 mL (not infrequently, <150 mL) and cannot be detected by abdominal percussion. Ultrasound is a useful and rapid means of determining bladder capacity. Voiding often can be triggered by stimulation of the skin of the abdomen, thigh, or genitalia, often with spasm of the lower extremities.
With high thoracic and cervical lesions, distention of the bladder (due to a plugged catheter or during cystometry or cystoscopy) can trigger a series of responses, including hypertension, bradycardia, headache, piloerection, and sweating (autonomic dysreflexia). Inserting a catheter and leaving the catheter on open drainage usually reverses the dysreflexia.
Virtually all patients experience one or more urinary tract infections during the recovery phase of spinal shock. This is due to the necessity of catheter drainage, either intermittent or continuous. Urinary stasis, prolonged immobilization, and urinary tract infections can predispose to stone formation. Renal function may be normal or impaired, depending on the efficacy of treatment and the absence of complications (hydronephrosis, pyelonephritis, urinary stones). Red blood cells (erythrocytes) in the urine may reflect a number of abnormalities. Uremia will result if complications are not addressed appropriately and the patient is not checked at regular intervals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree