Nephrotic syndrome results from urinary loss of albumin and other plasma proteins and is characterized by hypoalbuminemia, hyperlipidemia, and edema. Quantifying the loss of tissue protein is more difficult than measuring the urinary loss of albumin and other plasma proteins. However, marked muscle wasting (sometimes obscured by edema) occurs in patients with continuous proteinuria. In addition, micronutrients such as vitamin D, iron, and zinc are bound to plasma proteins that are lost, so depletion syndromes can occur when proteinuria is massive and continuous. Lipid metabolism is abnormal in the nephrotic syndrome resulting in hyperlipidemia and potentially, accelerated atherosclerosis and renal failure.
The major rationales for modifying a patient’s diet, then, are to blunt manifestations of the syndrome (such as edema and hyperlipidemia), to replace nutrients lost in the urine, and to reduce risks for progressive kidney disease and atherosclerosis. It should be mentioned that specific allergens in food may cause kidney disease in some patients, and dietary modification might be curative.
DIETARY PROTEIN
Metabolic abnormalities in nephrotic syndrome include depletion of plasma and tissue protein pools. Nephrotic syndrome in this case, resembles protein-calorie malnutrition (i.e., kwashiorkor) because in both cases, the plasma albumin concentration is reduced, plasma volume is expanded, and albumin pools shift from the extravascular to the vascular compartment. In protein-calorie malnutrition, providing the needed protein and calories will correct all of the manifestations of malnutrition. This is not the case in patients with the nephrotic syndrome.
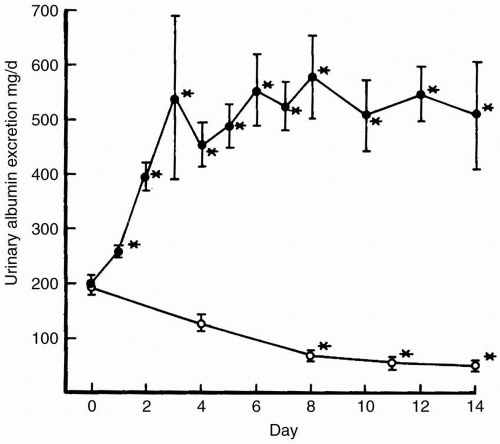
In patients with nephrotic syndrome, there are several causes of hypoalbuminemia: (i) albumin loss in the urine; (ii) an inappropriate increase in the fractional catabolic rate of albumin; and (iii) an insufficient increase in the synthesis rate of albumin to replace the loss. Placing patients with nephrotic syndrome on a high protein diet might seem reasonable in light of the massive urinary protein and amino acid losses. However, while average values for urinary protein losses are approximately 6 to 8 g per day (the amount contained in a hen’s egg), simply increasing dietary protein is of little benefit. This strategy is not recommended because dietary protein and, specifically, certain amino acids in the diet, increase glomerular permselectivity, i.e., an increase in the defect in the filtration barrier of the glomerular capillary
when compared with low-protein diets; filtration barrier defects result in increased urinary protein losses (
Fig. 8-1). This increase in permselectivity increases urinary protein loss and results in a net decrease in plasma protein mass despite any increase in albumin synthesis because of augmentation of dietary protein. Indeed, high protein diets likely contribute to loss of kidney function.
Conversely, dietary protein restriction in contrast, has several benefits in patients with chronic kidney disease (CKD) with nephrotic syndrome. There is reduced urinary protein excretion and plasma fibrinogen levels, decreased protein degradation and amino acid oxidation with improvement or achievement in neutral nitrogen balance, and a potential salutary effect on progressive loss of kidney function.
Reducing urinary protein excretion, regardless of how it is achieved, is desirable for several reasons. In addition to its beneficial
effect on protein metabolism, suppressing urinary protein excretion reduces the blood lipid levels, because the degree of hyperlipidemia varies directly with urinary protein losses. Proteinuria itself damages the renal interstitium directly through a variety of putative mechanisms. Filtered proteins are reabsorbed by the tubule and carry iron, complement components, and biologically active lipids into the interstitial space. Once lipids reach the interstitial space, they may act as chemoattractants for monocytes and cause renal injury. Reabsorbed iron from proteinuria is biologically active and may act as an oxidant to injure the kidney. In addition, diets that are high in protein also are high in acid content, leading to acidosis with increased ammoniagenesis by the kidney. The accelerated rates of renal ammonia production also may lead to renal injury. The reduction in urinary protein excretion that follows the initiation of a low-protein diet can have a potentially salutary effect on progressive renal injury through any or all of these mechanisms (
Table 8-1).
Several studies have suggested that the composition of proteins in the diet may be as important as its absolute nitrogen content. In experimental studies in rats, dietary augmentation of certain amino acids causes a prompt increase in urinary albumin excretion (UAE), whereas branched-chain amino acid supplementation does not affect proteinuria. Studies in animal models of nephrotic syndrome and in humans with CKD also suggest that specific types of dietary proteins may be particularly important. When patients with nephrotic syndrome were fed a vegetarian soy diet, urinary protein excretion decreased, as did blood lipid levels. In rats with puromycin-induced nephrotic syndrome, not only did a 20% soy protein diet lower proteinuria and blood lipid levels, but it also improved the creatinine clearance and decreased glomerular sclerosis while reducing the level of proinflammatory cytokines in the kidney. Because the soy protein diet was low in fat (28% of calories) and protein (0.71 g per kg ideal body weight), distinguishing whether the beneficial effects of a soy-based diet in patients with nephrotic syndrome from a special amino acid composition alone or from the lower fat and protein content is difficult. Studies of nephrotic rats suggested, however, that much of the benefit of a soy-based diet derives from a direct effect of soy proteins on the kidney (possibly through reducing the degree
of nitrotyrosine formation), rather than through changes in hepatic lipid metabolism. Curiously, urinary protein excretion differed only slightly when diets containing 1.1 g were compared with those containing 0.7 g soy protein/kg/day.
More recent animal studies suggest that a flaxseed-based protein diet may offer even more benefit than soy protein. In a study of the effects of 20% casein, 20% soy protein, or 20% flaxseed meal in a rat model of diabetic nephropathy using the obese spontaneously hypertensive rat model, flaxseed meal reduced proteinuria and glomerular and tubulointerstitial injury more than a casein- or soy protein-based diet; creatinine clearance and plasma creatinine levels did not differ. The common pathway for the beneficial effects of plant-protein diets may be the phytoestrogens that are present in soybeans as isoflavones and in flaxseed as lignans. These compounds are estrogen-like but how they affect the kidney is unknown. Plant-protein diets are high in polyunsaturated lipids, including α-linolenic acid in flaxseed. Polyunsaturated fats can improve the lipid profile and may reduce kidney injury. However, mechanisms for these responses are unknown.
In nephrotic patients, concomitant use of an angiotensin-converting enzyme (ACE) inhibitor with a high-protein diet (1.3 to 1.6 g protein/kg/day) prevented the diet-induced increase in urinary protein excretion. Serum protein and albumin levels increased significantly in eight patients after they crossed over to the high-protein diet with ACE inhibitor, compared with results with a mildly protein-restricted diet (0.8 g per day). The use of ACE inhibitor or angiotensin receptor blocker (ARB) in patients with normal to moderately restricted protein intake (0.8 g per day), in contrast, lowered levels of proteinuria, triglycerides, total cholesterol, and low-density lipoprotein (LDL) cholesterol. Recent data suggest that more complete blockade of the renin-angiotensin-aldosterone system (RAAS) with a combination of ACE inhibitor and ARB or addition of aldosterone inhibitor to the ACE inhibitor or ARB regimen is even more effective in reducing proteinuria. Unfortunately, hyperkalemia may develop. Whether protein restriction in addition to RAAS blockade offers further benefit is debated.
MICROALBUMINURIA
Although the focus of this chapter is on patients with nephrotic proteinuria, a much larger numbers of patients have low levels of urinary albumin loss (i.e., microalbuminuria). Microalbuminuria is found in hypertensive and diabetic subjects and predicts progression of kidney disease and cardiovascular risk in both populations. Microalbuminuria also increases the risk of cardiovascular disease even among patients who are not hypertensive or diabetic. The risk for developing urinary albumin excretion (UAE) in hypertensive diabetic subjects is related to the fraction of total calorie intake that is composed of dietary protein. Restriction of protein intake reduces UAE in both noninsulin-dependent and insulin-dependent diabetic subjects. The type of protein consumed also has an effect on UAE in diabetic subjects, suggesting
that either the amino acid composition or the lipid composition of the food ingested contributes to the effect of a protein-rich meal.
Recommendations
Because urinary protein excretion varies from day to day in individual patients, we recommend collecting 3 separate 24-hour urine specimens, with simultaneous measurements of serum albumin and protein, to establish baseline values. Patients are then placed on a 35-kcal per kg diet that contains 0.8 to 1.0 g protein per kg and is restricted to 2 g of sodium. We then monitor 24- hour urinary urea excretion every 2 to 3 months to ensure that patients are not eating more (or less) protein than recommended. The goal is to decrease proteinuria without reducing serum albumin and protein concentration. This goal is usually attainable when dietary protein intake is restricted to these levels (
Table 8-2).
Dietary protein intake can be estimated from nitrogen excretion measured in the steady state. The steady-state nitrogen excretion (previously abbreviated as the protein catabolic rate or PCR) is principally determined by the urea nitrogen excretion. Therefore, if total body urea pools are constant (i.e., the blood urea nitrogen [BUN] and weight are stable), then the amount of protein ingested can be estimated from the formula:
Protein intake = [0.031 gN/kg/day]body weight in kilograms/0.16 + [24-hour urine urea nitrogen excretion/0.16] g/day + 24-hour urine protein excretion g/day (see
Chapter 6)
If the estimated protein intake by this method varies from the amount of protein prescribed for the diet, the patient should be referred to the nutritionist/dietician.
Although “high-quality” protein (meat and dairy products) contain a higher fraction of essential amino acids (EAA), vegetarian diets based on soy protein are more effective in reducing urinary
protein loss, increasing serum protein levels, correcting hyperlipidemia, and reducing renal inflammation and fibrosis. Consequently, we recommend soy-based protein in the diet. A plant-based diet may be even more useful in preventing the development of diabetic kidney disease, in ameliorating the progression of diabetic nephropathy, and in preventing obesity-related kidney diseases. An ACE inhibitor and/or ARB should be used concomitantly to reduce urinary protein losses.