Fig. 35.1
Overall survival comparison for NACT in surgical setting from NACCMA Collaboration meta-analysis (From Tierney et al. [13]; used with permission from Elsevier)
In 2007 the Gynecologic Oncology Group (GOG) published the results of GOG-141. This trial randomized 288 patients (over 5 years) with stage IB2 cervical cancer to NACT using three cycles of cisplatin and vincristine given every 10 days followed by surgery or surgery alone. This trial was closed early due to poor accrual and frequent off protocol use of post operative radiotherapy (45 % of NACT patients and 52 % of surgery alone). Response rates as defined by ‘clinical objective response’ to NACT were 52 % but no significant differences were seen in pathological findings, progression free survival (PFS) or overall survival [14].
Mossa et al. demonstrated similar results having randomized 288 patients with FIGO stage IB to III cervical cancer to NACT with three cycles of cisplatin, vincristine and bleomycin versus conventional treatment (surgery or radiotherapy alone). For the vast majority of these patients (258) the conventional treatment was surgery and the remaining 30 were stage III patients who received radiotherapy (6 in NACT group, 24 in conventional group). At 7 year follow up no statistically significant benefit in disease free or overall survival was seen but the trend was favoring NACT, OS 70.4 % NACT versus 65.9 %, DFS 65.4 % versus 53.5 % [15].
More recently two randomized trials, SNAP-01 and SNAP-02, showed that paclitaxel containing regimens are associated with improved response rates. Paclitaxel/ifosfamide/cisplatin (TIP) or paclitaxel/epirubicin/cisplatin (TEP) yielded optimal response rates (no residual or <3 mm stromal invasion) of 42–48 % but at the expense of significant hematological toxicity [9].
A number of phase two studies have investigated the platinum/paclitaxel doublet given in a dose dense schedule (cycles <10 days) to patients with FIGO stage IB-IIB disease prior to surgery [16, 17]. Although these are small trials response rates of up to 90 % were observed with a short course of dose dense chemotherapy without the significant toxicity observed in the Italian studies above.
Three cycles of neoadjuvant carboplatin and paclitaxel given every 21 days was used by Duenas-Gonzalez et al. with even more impressive response rates of 95 % in 43 patients with FIGO IB2-IIIB cervical cancer, but this was assessed by clinical response rather than MRI [18].
In 2010 a Cochrane review comparing NACT and surgery versus surgery alone in FIGO stage IB to III was published. Six randomized controlled trials involving over 1,000 women were identified and whilst data for PFS was available for all trials, overall survival, resection rates and pathological response data was not available for one trial. Recurrence data was only available for three trials. Therefore the final analysis was limited to five trials with 604 women. This review concluded that NACT before surgery was associated with an improvement in PFS (HR 0.76 p = 0.01) whilst there was a trend towards an improvement in overall survival this did not reach statistical significance (HR 0.85, p = 0.17). Once again, these trials were very heterogeneous and results were confounded by the frequent use of radiotherapy in addition to the chemotherapy and surgery [19].
A further meta-analysis of five randomized controlled trials and four observational studies involving 1,784 patients with FIGO IB1 to IIA was published in 2013 [20]. The authors concluded that NACT, whilst decreasing tumor bulk and lymph node metastases, did not improve survival. They did note that postoperative radiotherapy was less frequently indicated. However, these findings need to be interpreted with caution due to the limitations of the review and the inclusion of observational studies.
The ongoing European Organization for Research and Treatment of Cancer (EORTC 55994) randomized phase three trial may help to define the role, if any, of NACT in early stage disease. This compares the use of NACT followed by surgery with concurrent chemo-radiotherapy for FIGO stage IB2-IIB cervical squamous cell carcinoma, adenosquamous or adenocarcinoma. The NACT regimen typically consists of three cycles of 21 day cisplatin based combination chemotherapy. Overall survival is the primary endpoint with PFS, toxicity, and Quality of Life as secondary endpoints. However the trial has been open to recruitment since 2002 and accrual has been slow (Table 35.1).
Table 35.1
Summary of trials investigating NACT in the surgical setting
Author | Year | No of pts | NACT regimen | Outcome |
|---|---|---|---|---|
Sardi et al. | 1997 | 210 | 3× cisplatin 50 mg/m2, vincristine 1 mg/m2, bleomycin 25 mg/m2 (day 1–3) q10 | At 67 months |
DFS 80 % vs 61 % | ||||
Eddy et al. | 2007 | 288 | 3× cisplatin 50 mg/m2, vincristine 1 mg/m2, q10 | Clinical objective RR 52 % |
No sig diff in path, PFS, OS | ||||
Mossa et al. | 2010 | 288 | 3× cisplatin 50 mg/m2, vincristine 1 mg/m2 bleomycin 25 mg/m2 | At 7 years |
OS 70.4 % vs 65.9 % (not sig) | ||||
DFS 65.4 % vs 53.5 %(not sig) | ||||
Park et al. | 2004 | 43 | 3× cisplatin 60 mg/m2, paclitaxel 60 mg/m2 q10 | Clin response 90.7 % (MRI&exam) |
Path downstaging 72.1 % | ||||
Mori et al. | 2008 | 30 | 6× carboplatin AUC2, paclitaxel 60 mg/m2 q7 | Objective RR 87 % (MRI&exam) |
Duenas Gonzalez et al. | 2003 | 43 | 3× carboplatin AUC6, paclitaxel 175 mg/m2 q21 | Clinical RR 95 % (MRI) |
35.4 Neoadjuvant Chemotherapy Before Radiotherapy/Chemo-Radiotherapy
A meta-analysis of individual patient data from 21 randomized trials comparing NACT before radiotherapy versus radiotherapy alone was published in 2003 [13]. The final analysis included data from 18 trials with a total of 2,074 patients. Overall the analysis concluded that NACT had no significant impact on overall survival. However, heterogeneity in chemotherapy cycle length and platinum dose intensity were identified as important factors in determining outcome. Trials using a short cycle length (<14 days) gave a pooled HR of 0.83, equivalent to a 7 % improvement (45–52 %) in 5 year survival (see Fig. 35.2). In contrast, those trials that used longer cycle lengths (>14 days) gave a pooled HR of 1.25 equivalent to an absolute detriment in survival of 8 % (45–37 %) at 5 years. Platinum dose intensities >25 mg/m2 were also associated with better outcomes. Furthermore, there was significant variation in radiation dose (both external beam and brachytherapy with the total doses delivered in the range 55–80 Gy) and the interval between completing chemotherapy and commencing radiotherapy. It is possible that the detrimental effect of longer chemotherapy cycle length is due to accelerated tumor regrowth in a tumor that is known to have a high growth fraction and proliferation rate. It is also important to note that this meta-analysis excluded trials using concurrent chemo-radiotherapy which is now the standard of care (Table 35.2).
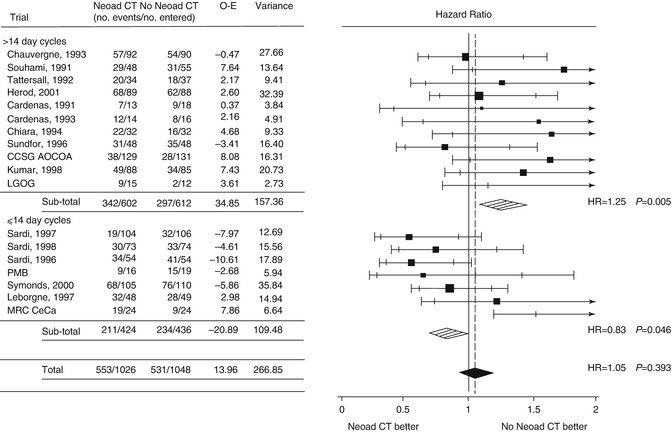

Fig. 35.2




Forrest plot demonstrating overall survival by planned chemotherapy cycle length from NACCMA Collaboration meta-analysis (From Tierney et al. [13]; used with permission from Elsevier)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








