Primary treatment of carcinoma of the esophagus and cardia rests on surgical resection. Although recent advances have shown the suitability of endoscopic treatment in selected patients with very early cancers, and preliminary studies have suggested that responders to primary chemoradiation may be equivalent to resection in selected patients with squamous cell carcinoma, surgical resection remains the mainstay of therapy, as it has for the past 50 years. Various changes support highly individualized treatment decisions, in which each patient receives the treatment with the best chance of eliminating all disease.
Primary treatment of carcinoma of the esophagus and cardia rests on surgical resection. Although recent advances have shown the suitability of endoscopic treatment in selected patients with very early cancers, and preliminary studies have suggested that responders to primary chemoradiation may be equivalent to resection in selected patients with squamous cell carcinoma, surgical resection remains the mainstay of therapy, as it has for the past 50 years.
National Cancer Institute statistics report that approximately 16,460 people will be diagnosed with esophageal cancer in 2008, and 14,280 or 87% are expected to die from it. These figures increase substantially when data for cancer of the cardia are included. Clearly, curing this disease is a challenge. The clinical biology and presentation of esophageal cancer have changed dramatically in the past 25 years. This has had significant impact on the intent of treatment, resulting in a marked trend favoring curative therapy whenever possible. The liberal use of flexible endoscopy to investigate foregut symptoms and the widespread adoption of Barrett’s surveillance programs have markedly increased the proportion of patients in which the cancer is found when the disease may be confined to the mucosa or submucosa. Trends also document an increasing prevalence of adenocarcinoma of the esophagus in younger patients. Large series of those less than 50 years old have been reported. The morbidity and mortality associated with esophageal resection has declined such that dedicated centers routinely achieve 1% to 2% 30-day mortality. Taken together, these changes support highly individualized treatment decisions in which each patient receives the treatment with the best chance of eliminating all disease.
Assessment of the extent of disease and selection of surgical options
At the initial encounter with a patient diagnosed as having carcinoma of the esophagus, a decision must be made as to whether he or she is a candidate for curative surgical therapy, palliative surgical therapy, or nonsurgical palliation. The pros and cons of the use of adjuvant therapy must also be considered, and its timing. Making these judgments can be difficult. Current methods evaluating the pretreatment stage of esophageal carcinoma are imprecise, primarily because of the difficulty of measuring the depth of tumor penetration of the esophageal wall and the inaccessibility of the organ’s widespread lymphatic drainage. Even with the modern techniques of CT, MRI, positron emission tomography (PET), endoscopic ultrasound (EUS), and laparoscopic and thoracoscopic technology, pretreatment staging remains inaccurate in many patients.
Esophageal cancer generally presents with dysphagia, although increasing numbers of relatively asymptomatic patients are now identified on surveillance endoscopy or with endoscopy prompted by nonspecific upper gastrointestinal symptoms. Portale and colleagues found a surprisingly high proportion of 213 patients referred for surgical resection before the development of dysphagia ( Table 1 ). Twenty-five percent of the 213 patients were enrolled in a surveillance program for Barrett’s esophagus or presented with a long history of gastroesophageal reflux disease symptoms, and in another 30% occult bleeding, anemia, or abdominal symptoms, such as pain or discomfort, prompted the physician visit leading to a diagnosis of cancer ( Table 2 ). Dysphagia usually presents late in the natural history of the disease and becomes severe only when more than 60% of the esophageal circumference is infiltrated with cancer. Consequently, the cancer is often systemic by the time dysphagia heralds its presence.
| N = 213 | |
|---|---|
| Main symptom at presentation a | |
| Nondysphagia | 121 (56.8) |
| Barrett’s surveillance or gastroesophageal reflux disease | 44 (24.4) |
| Bleeding (anemia, hematemesis) | 29 (13.6) |
| Chest and abdominal pain, discomfort | 26 (12.2) |
| Others | 14 (6.6) |
| Dysphagia | 92 (43.2) |
| Duration of dysphagia before diagnosis (mo) b | 2 (1–3) |
| N = 184 | Early Stage (PPV) | Lymph Node + | |
|---|---|---|---|
| No dysphagia as main symptom at diagnosis | 95 | 51.6 | 42.1 |
| Tumor length ≤2 cm | 67 | 73.1 | 20.9 |
| CI ≤25% | 62 | 79 | 17.7 |
| No dysphagia+tumor length ≤2 cm | 55 | 80 | 14.5 |
| No dysphagia + CI ≤25% | 55 | 80 | 16.4 |
| Tumor length ≤2 cm + CI ≤25% | 56 | 80.4 | 16.1 |
| No dysphagia + tumor length ≤2 cm + CI ≤25% | 50 | 82 | 14 |
The characteristics of esophageal cancer that are associated with improved survival are known. Most studies suggest that only two factors (metastasis to lymph nodes and tumor penetration of the esophageal wall) have significant and independent influence on prognosis. The beneficial effects of the absence of one factor persist even when the other is present. Factors known to be important in the survival of patients with advanced disease, such as cell type, degree of cellular differentiation, or location of tumor in the esophagus, have no effect on survival of patients who have undergone resection for early disease. Although current staging systems fail to take the number of lymph nodes into account, most studies also show that patients having five or less lymph node metastases have a better outcome.
Clinical factors that indicate an advanced stage of carcinoma and exclude surgery with curative intent are recurrent nerve paralysis, Horner’s syndrome, persistent spinal pain, paralysis of the diaphragm, fistula formation, and malignant pleural effusion. Factors that make surgical cure unlikely include a tumor greater than 8 cm in length, abnormal axis of the esophagus on a barium radiogram, enlarged paratracheal or para-aortic lymph nodes on CT, a weight loss more than 20%, and loss of appetite. In patients where these findings are not present, staging depends primarily on the length of the tumor as measured with endoscopy, and the degree of wall penetration and lymph node metastasis seen with EUS or other staging modalities. Studies indicate that there are a high number of favorable parameters associated with tumors less than 4 cm in length, there are fewer with tumors between 4 and 8 cm, and favorable criteria for tumors greater than 8 cm in length are uncommon.
EUS has gained popularity over the last 10 years as the modality of choice for pretherapeutic assessment of depth of tumor infiltration into the esophageal wall and lymph node status. Although it provides the best information available regarding both of these important staging parameters, recent data reveal major limitations particularly in differentiating mucosal and submucosal tumors, a key branchpoint in therapeutic decision making. The preoperative locoregional lymph node staging accuracy is improved, however, by performing EUS-guided fine-needle aspiration. Nishimaki and colleagues prospectively assessed the accuracy of preoperative staging using esophagography, esophagoscopy, EUS, and CT in 224 patients with localized esophageal cancer who underwent radical esophagectomy. The study showed that T2 tumors were poorly predicted by endoscopy or esophagography and even though EUS was shown to be the best study for predicting T2 tumors, its accuracy was only 61%, with 33% of patients being overstaged. The overall accuracy of correctly predicting stage I disease before esophagectomy was only 68% and at predicting stage III disease was 70%. The accuracy of clinical staging for predicting stage IIA or IIB disease was less than 50%.
The limits of EUS are particularly evident in the attempt to distinguish intramucosal from submucosal tumors and submucosal from intramuscular ones. In a study from the Cleveland Clinic Foundation, Zuccaro and colleagues directly compared clinical TNM classification with pathologic classification in 266 patients with esophageal cancer who had EUS staging followed directly by esophagectomy. EUS was confirmed to be reasonably accurate in the identification of T3 tumors (83%). T2 tumors were correctly identified by EUS in only 42% of patients; in 54% they were erroneously overstaged to T3. Even less accurate was the identification of T1 or in situ tumors. T1 lesions were correctly classified in 29% of patients and in situ tumors were never correctly identified preoperatively. T1 tumors were overstaged in 46% of cases and understaged in 24%. It is interesting to note that 16 patients with T1 tumors (20%) and 13 patients with Tis tumors (68%) were misdiagnosed as clinical T0.
Endoscopic mucosal resection (EMR), initially used as a definitive resection option for early esophageal cancer and high grade dysplasia, has recently been proposed as a staging tool to determine the depth of invasion of esophageal adenocarcinoma and may become the procedure of choice reliably to differentiate intramucosal from submucosal lesions. Mino-Kenudson and colleagues from Massachusetts General Hospital found that interobserver agreement of EMR for Barrett’s esophagus–related superficial neoplasms is significantly higher than for mucosal biopsies.
PET and particularly PET-CT scanning are emerging as both a highly useful staging modality and also as a technique that provides prognostic information, such as predicting the response to neoadjuvant therapy. In a recent meta-analysis, van Vliet and colleagues assessed the diagnostic accuracy of EUS, CT, and PET. The results suggest that each modality plays a distinctive role in staging of esophageal cancer. For detection of regional metastases, EUS seems to be the most sensitive (0.80 versus 0.50 and 0.57 pooled sensitivity with CT and PET, respectively) and PET the most specific (0.85 versus 0.83 and 0.70 pooled specificity for CT and EUS, respectively) diagnostic procedure. For distant metastases combined CT-PET has significantly higher specificity and sensitivity than CT.
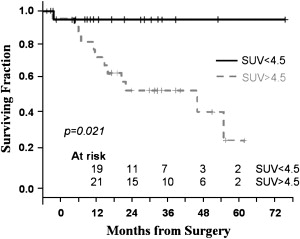
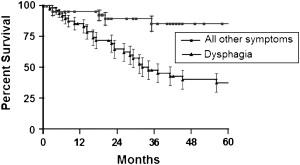
Rizk and colleagues from the Memorial Sloan-Kettering Cancer Center evaluated the significance of preoperative PET standardized uptake values (SUV) in predicting survival of 55 patients with adenocarcinoma of the distal esophagus and gastroesophageal junction who underwent esophagectomy alone as primary treatment of their disease. Patients with low SUV less than 4.5 were more likely to have early stage disease, T1-2 disease, and less lymph node involvement than patients with high SUV (90% versus 60% and 8% versus 48%). The overall survival in the low SUV group was significantly better than in the high SUV group ( Fig. 1 ). Furthermore, within the subset of patients with early clinical or pathologic stage disease, patients with low preoperative SUV had a significantly better survival. When the SUV was considered as a continuous variable, it correlated with the presence of lymph node metastasis, the number of nodes involved, and the risk of death. The authors concluded that PET SUV could be used to predict clinical or pathologic stage and overall survival in patients with esophageal cancer. High PET SUV also identifies patients with poor prognosis from within a group of patients that would otherwise be considered to have curable early stage disease and could be potentially used to stratify patients for appropriate therapy.
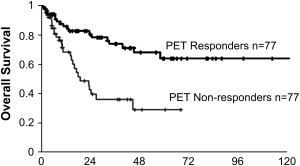
PET has also been proposed as a valuable tool to predict response to preoperative chemotherapy. Investigators from Munich, Germany, reported the results of the MUNICON trial on the use of [ 18 F]fluorodeoxyglucose PET as a predictor of response and patient survival in patients with locally advanced adenocarcinoma of the distal esophagus or cardia (T3NxM0) treated with preoperative chemotherapy. These data suggested that a decrease of tumor metabolic activity (SUV) by more than 35% after 2 weeks of therapy predicts a high pathologic response rate and is associated with a better survival. Among 110 patients evaluated with [ 18 F]fluorodeoxyglucose PET at baseline and 14 days after chemotherapy was started, 49% of patients were classified as metabolic responders based on tumor uptake and were offered full-dose chemotherapy and then proceeded to surgery. In nonresponders chemotherapy was discontinued and they went directly to surgery. Metabolic response was highly associated with clinical and histopathologic response. Overall survival of metabolic responders was significantly better than nonresponders ( Fig. 2 ). Among the 104 patients who underwent surgical resection after completion of neoadjuvant chemotherapy, metabolic response remained a significant prognostic factor.
An individualized surgical approach
At diagnosis, cancer must be either local (confined to the mucosa or submucosa and node negative); regional (node positive without systemic metastasis); or systemic ( Box 1 ). It is reasonable to conclude that the extent of operation has little effect on survival of systemic disease but it can have a critical impact on survival for patients with isolated regional disease. The existence of a population of patients with regional disease has been debated in cancer biology, but can be proved by the extensive documentation that cure is possible with surgical resection alone in the presence of positive lymph nodes. The relatively small size of this group and the difficulty in clinically identifying these patients preoperatively account for the difficulty in establishing the benefit of lymphadenectomy as an adjunct to surgical resection.
Local disease: (15%)
Asymptomatic
Minimal or no visible lesion
Regional disease: (25%)
Minimal symptoms (anemia)
Small (<2–3 cm) noncircumferential
Systemic disease: (60%)
Dysphagia
>3 cm circumferential tumor, plus nodes on EUS
Patient with a High Probability of Disease Confined to the Mucosa (Localized Disease)
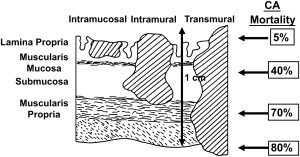
Treatment decisions, efficacy, and importantly prognosis are all highly dependent on making the distinction between dysplasia, intramucosal, and submucosal cancer to the extent possible. Intraepithelial tumors (HGD, carcinoma in situ) have a quite different biologic behavior than intramucosal tumors, which are very different than submucosal tumors ( Fig. 3 ). Vascular invasion and lymph node metastasis do not occur in HGD and carcinoma in situ and are uncommon in intramucosal tumors, but occur in more than one quarter of patients with submucosal lesions. As a consequence, the 5-year survival for intramucosal tumors is significantly better than a tumor that has invaded the submucosa. Techniques of curative resection given a high probability of local disease range from endoscopic resection and ablation to standard operative esophagectomy.
Endoscopic treatment
EMR, photodynamic therapy, radiofrequency ablation, and combinations of these have been studied in the setting of HGD and early invasive carcinoma. Photodynamic therapy suffers from a high prevalence of incomplete ablation; a relatively high (30%) prevalence of strictures; and lack of availability and expertise in many locations. As such its application is becoming much less common. EMR has taken its place and in the proper setting is a very viable treatment option, particularly when associated with the addition of radiofrequency ablation. The primary limitation of EMR is the need to “see” the area of interest. Local recurrence or development of metachronous lesions after EMR has been reported in 15% to 30% of patients. The combination of local resection and ablation has been used to reduce this possibility, especially in high-risk patients who may be precluded from undergoing esophagectomy because of multiple comorbidities.
The largest experience to date combining endoscopic techniques in the treatment of early neoplasia has been reported by investigators in Wiesbaden, Germany. The outcome of 100 consecutive patients with low-risk adenocarcinoma of the esophagus arising in Barrett’s esophagus patients who underwent EMR was assessed. A total of 144 resections (1.47 per patient) were performed without technical problems. No major complications and only 11 minor ones (bleeding without decrease of Hb >2 g/dL, treated with injection therapy) occurred. Complete local remission was achieved in 99 of the 100 patients after 1.9 months (range, 1–18 months) and a maximum of three resections. During a mean follow-up period of 36.7 months, recurrent or metachronous carcinoma was found in 11% of the patients, but successful repeat treatment with EMR was possible in all of these. The calculated 5-year survival rate was 98%. This report established EMR as a safe and efficacious treatment in highly select patients with HGD and intramucosal adenocarcinoma. In a follow-up publication, results after 5-year follow-up in 349 patients with high-grade intraepithelial neoplasia and intramucosal adenocarcinoma were reported. Endoscopic resection was performed in 279 patients, photodynamic therapy in 55, and both in 13. A complete response was achieved in 96% of the patients; 3.7% required surgery for failed endoscopic treatment. Metachronous disease developed in 21% and the calculated 5-year survival rate was 84%. Risk factors associated with recurrence included piecemeal resection, long-segment Barrett’s, no ablative therapy after mucosal resection, multifocal neoplasia, and a time to complete resection of more than 10 months.
The context of the use of EMR is critical, however, and it is important that these data not be extrapolated in the treatment of patients beyond those described in these studies. The described outcomes require a highly selected group of patients, which may be difficult for most centers to reproduce. The 100 patients reported from Wiesbaden were selected out of 667 possible candidates over 7 years. This equates to the referral of nearly 100 patients with very early esophageal adenocarcinoma per year. Most centers see a handful at best. All patients underwent very intensive staging, including EUS and radiographic procedures; high-resolution videoendoscopy with methylene blue chromoendoscopy; detailed morphologic assessment of the lesions according to the Japanese classification for early gastric cancer; an intense biopsy protocol (four quadrants, every 1 cm); routine assessment by two different pathologists; and high-frequency (20 MHz) ultrasound. Equally intense follow-up was required with endoscopy at 1, 2, 3, 6, 9, 12, 16, 24, 30, and 36 months with repeated high-resolution and chromoendoscopy, a routine rigorous biopsy protocol, EUS and abdominal ultrasound, and CT. The rigor of the patient selection should be evident and the difficulty most would have in reproducing it. The persistent neoplastic risk in the remaining Barrett’s mucosa is also a concern.
Surgical resection
Options for esophageal resection in early stage cancer include transhiatal or transthoracic simple esophagectomy, vagal-sparing, or minimal invasive esophagectomy. The chance of curative resection with a simple esophagectomy is critically dependent on accurate identification of the location and depth of the tumor or the presence of regional nodal metastases. Because the perfect tool for preoperative staging of esophageal cancer is not yet available, the presence or absence of an endoscopically visible lesion in patients with HGD or intramucosal carcinoma has been proposed as a predictor of tumor depth and nodal metastases. The data indicate that a positive biopsy in the absence of an endoscopically visible lesion almost always corresponds to an intramucosal tumor without nodal metastases. In addition, a number of studies have shown that about 40% to 50% of patients with the preoperative diagnosis of HGD were indeed proved to harbor occult adenocarcinoma at resection. EMR may be the best method to stage the depth of invasion of small esophageal tumors. Currently, patients with documented, confirmed HGD or adenocarcinoma in the absence of a visible endoscopic lesion are appropriate candidates for vagal-sparing or minimally invasive esophagectomy.
Vagal-sparing esophagectomy
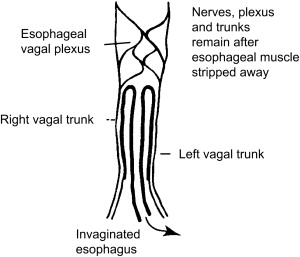
The technique of vagal-sparing esophagectomy was developed by Akiyama and coworkers to avoid the functional morbidity of standard esophagectomy by preserving the vagal trunks while removing all at-risk mucosa ( Fig. 4 ). This approach is suitable only given confidence of the absence of regional nodal disease. The operation can be done with either open or laparoscopic techniques. The vagal nerves are identified and the proximal lesser curve is dissected similar to a highly selective vagotomy. Vessel loops are placed around the tissue bundles including both nerves. A gastric conduit is prepared and the stomach is divided below the esophagogastric junction. The left neck is dissected in a standard fashion exposing the cervical esophagus and it is divided at the level of thoracic inlet. A standard vein stripper is passed through the esophagus from abdomen to neck and allowed to exit from the cut end of the cervical esophagus. A blunt “knob” is threaded onto its end and the esophagus is secured around the stripping device using an Endoloop. The stripper is slowly and gently pulled from the abdominal site progressively inverting the esophagus stripping it out of the posterior mediastinum. This maneuver strips the mucosa, circular, and most of the longitudinal muscle layer but preserves the vagal plexus intact. Gastrointestinal continuity is restored by a gastric pull-up or colon interposition.
Banki and colleagues from University of Southern California reported their initial results of vagal-sparing esophagectomy. Vagal function, assessed by increased acid output and pancreatic polypeptide levels after sham feeding and a preservation of normal gastric emptying, remained intact in 70% of the patients. Follow-up publications showed that the length of hospital stay and the incidence of major complications were significantly reduced when compared with transhiatal or en bloc transthoracic resection. Importantly, postvagotomy symptoms, such as dumping and diarrhea, were less common in the vagal-sparing group. Jobe and colleagues reported minimally invasive vagal-sparing methods ( Fig. 5 ).
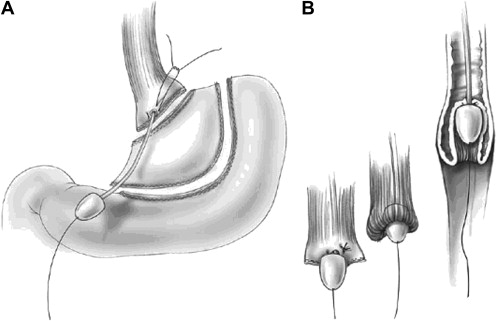
Candidates for vagal-sparing resection are relatively few and include those with high-grade dysplasia or nonvisible invasive cancer. The rationale includes the need for life-long, meticulous, and intensive follow-up required after endoscopic techniques and the small but real prevalence of failure, synchronous and metachronous cancer. Vagal-sparing esophagectomy has the obvious advantage of removing all at-risk mucosa, which obviates the need for rigorous follow-up.
Minimal invasive esophagectomy
As in other fields of surgery, with the technical advances of the recent years, minimal invasive esophagectomy became feasible and gained popularity, although concerns have been expressed about its safety and oncologic outcomes. Several methods are available, depending on the chosen combination of abdominal and thoracic procedures. Most published studies are retrospective case series, case reports, and reviews. A recent systematic review of 23 articles on minimal invasive esophagectomy concluded that the available literature is hampered by selection and publication bias. Because direct comparisons with open surgery are lacking (most of the results are compared with historical data from open series), the potential benefit of minimally invasive surgery remains unclear.
Patient with a High Probability of Regional Disease
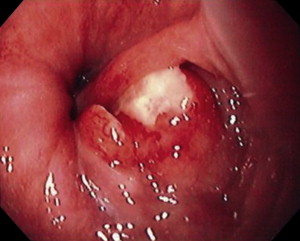
Although by no means certain, the probability of curable disease limited to regional nodes is high given a patient presenting with or without dysphagia ( Fig. 6 ) who on endoscopy has a small (<1–2 cm) noncircumferential visible tumor ( Fig. 7 ). It is in this setting that invasion through the muscularis mucosa into the submucosa is likely, along with a 25% or more chance of lymph node metastasis, but a reasonable chance of no systemic spread. Evidence is growing that under these circumstances, which occur in as many as 15% to 25% of patients referred for surgical resection, resection and en bloc lymphadenectomy provides the best chance of cure and should be the treatment of choice ( Fig. 8 ). Supporting evidence was recently provided by the randomized study conducted at the University of Amsterdam by Omloo and colleagues in which the 5-year outcome of simple transhiatal and en bloc transthoracic resection for esophageal cancer was reported. Patients with more than one, but less than eight positive nodes benefited from the extended lymphadenectomy. Although no statistical difference in the overall 5-year survival rates was found, subgroup analysis identified that patients with between one and eight positive nodes had significantly improved long-term survival.
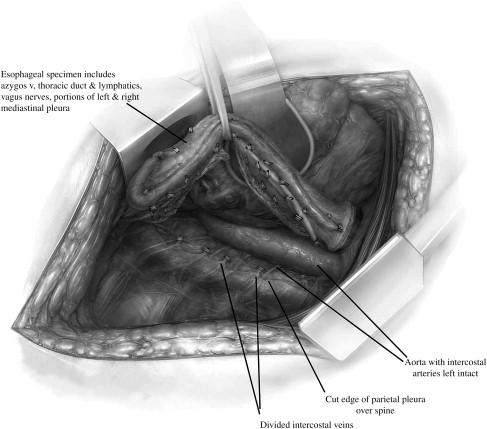
The primary rationale for an en bloc resection and extended lymphadenectomy is to minimize the incidence of local and regional recurrences and maximize the chances of long-term survival and cure ( Table 3 ). Local recurrence has been shown to be very uncommon (<5%–10%) following en bloc resection, which contrasts with a 35% prevalence of local failure following transhiatal esophagectomy and a 15% to 20% prevalence after Ivor-Lewis resection ( Table 4 ). These findings may be explained by the removal of unrecognized, often microscopic, disease with more extensive lymph node dissection. Involved nodes that are left behind during simple esophagectomy are likely the cause of recurrent local disease.
| Surgical Resection | N | % Local Recurrence within Operative Field |
|---|---|---|
| En bloc a | 94 | 1 |
| Ivor-Lewis b | 100 | 14 |
| Transhiatal c | 144 | 35 |
| Chemotherapy + surgery d | 124 | 32 |
| Chemotherapy + radiotherapy + surgery e | 50 | 19 |
a Data from Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 2001;234:520–30 [discussion 530–1].
b Data from King RM, Pairolero PC, Trastek VF, et al. Ivor Lewis esophagogastrectomy for carcinoma of the esophagus: early and late functional results. Ann Thorac Surg 1987;44:119–22.
c Hulscher et al. J Am Coll Surg 2000,191:143–8.
d Data from Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979–84.
e Data from Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305–13.
| Author and Reference | No. of Subjects | Local Recurrence (%) |
|---|---|---|
| En bloc esophagectomy | ||
| Matsubara, et al | 171 | 10 |
| Altorki and Skinner | 111 | 8 |
| Hagen, et al | 100 | 1 |
| Collard, et al | 324 | 4 |
| Swanson, et al | 250 | 5.6 |
| Range | 1–10 | |
| Transhiatal esophagectomy | ||
| Hulscher, et al | 137 | 23 |
| Becker, et al | 35 | 31 |
| Gignoux, et al | 47 | |
| Nygaard, et al | 186 | 35 |
| Range | 23–47 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




