Gastroesophageal reflux disease (GERD) is defined by either subjective complaints indicative of problematic gastroesophageal reflux or objective complications directly attributable to reflux. Studies focused on GERD-related symptoms suggest a worldwide increase in prevalence of approximately 4% per year. Epidemiologic data indicate that possible contributing factors include increasing longevity, rising obesity rates, greater consumption of medications affecting esophageal function, and potentially changing prevalence rates of Helicobacter pylori infection. This article explores the interplay between pathophysiology and epidemiology by focusing on these risk factors and their potential effect on GERD pathogenesis.
Gastroesophageal reflux disease (GERD) is defined by either subjective complaints indicative of problematic gastroesophageal reflux or objective complications directly attributable to reflux. Although the precise incidence and prevalence of GERD are difficult to establish without a valid gold standard definition, studies focused on GERD-related symptoms suggest a worldwide increase in prevalence of approximately 4% per year. The cause of the increased GERD prevalence is debated and likely multifactorial. Epidemiologic data indicate, however, that possible contributing factors include increasing longevity, rising obesity rates, greater consumption of medications affecting esophageal function, and potentially changing prevalence rates of Helicobacter pylori infection. Although the association of GERD with these specific risk factors is insufficient to prove causality, each one is associated with a plausible mechanism for augmented esophageal acid exposure. This article explores the interplay between pathophysiology and epidemiology by focusing on these risk factors and their potential effect on GERD pathogenesis.
Prevalence of gastroesophageal reflux disease
Although there is substantial geographic variation in GERD prevalence, with very low rates in Africa and Asia and high rates in North America and Europe, overall prevalence seems to be increasing worldwide. A recent systematic analysis used a time-trend analysis with a Poisson regression model to assess GERD prevalence based on existing population-based studies, each using the prevalence of at least weekly heartburn or acid regurgitation as indicative of GERD. The model confirmed a statistically significant ( P <.0001) increase in the prevalence of GERD in the worldwide population; mainly in North America ( P = .0005) and Europe ( P <.0001), but not in Asia ( P = .49). In addition, most longitudinal studies reported an increase in the prevalence of GERD or esophagitis during the past two decades.
The etiology of this rise in prevalence is uncertain; however, epidemiologic data suggest that multiple factors may be responsible. To assess which of these are biologically plausible, one must first consider the pathophysiology of GERD and how potential risk factors may be related. The sections of this article are organized to focus first on GERD pathogenesis and, second, on how each specific risk factor may exacerbate pathophysiologic variables.
Pathophysiology of gastroesophageal reflux disease
Gastroesophageal reflux is a normal physiologic event, but excessive exposure of esophageal or supraesophageal epithelium to gastric refluxate resulting in either mucosal injury or related symptoms is the fundamental abnormality in GERD. The genesis of reflux injury and reflux symptoms are not the same but each relates to some combination on pathophysiologic factors leading to an excessive number of gastroesophageal reflux events, impaired clearance of refluxate from the esophagus, increased acidity of the refluxed gastric juice, or increased sensitivity of the esophageal or supraesophageal mucosa to that refluxate. Aberrations in one or more potential pathophysiologic factors can result in creation of conditions conducive to the development of esophagitis or such symptoms as heartburn. This shift from a compensated to a decompensated condition can be conceptualized as a dynamic balance between the aggressive forces promoting reflux, mucosal injury, and symptom generation and defensive forces that counteract these.
Antireflux Barrier
The primary line of defense against GERD is the integrity of the antireflux barrier. GERD, and all that it entails, cannot occur unless the antireflux barrier permits gastric juice to enter the esophagus. Localized at the esophagogastric junction (EGJ), the antireflux barrier is an anatomically complex zone whose functional integrity in preventing reflux has been variably attributed to intrinsic lower esophageal sphincter (LES) pressure, extrinsic compression of the LES by the crural diaphragm, the intra-abdominal location of the LES, integrity of the phrenoesophageal ligament, and maintenance of the acute angle of His promoting a “flap valve” function. The global function of the EGJ as an antireflux barrier is dependent on the sum of its constituent parts and their ability to maintain a high-pressure zone or a closed luminal segment in the region separating the stomach from the esophagus. The EGJ high-pressure zone is typically maintained during the interdeglutitive state by contraction of the LES and surrounding crural diaphragm.
An important distinction to make with respect to reflux is between EGJ relaxation, evident by the absence of a contractile pressure, and EGJ opening, wherein an intraluminal space is created. Relaxation is not always associated with opening. Furthermore, even with opening, the volume of ensuing reflux is strongly related to the dimensions of luminal opening that occurs. The volume of reflux that occurs following opening is strongly related to the opening dimensions of the EGJ, the pressure gradient between the gastroesophageal pressure gradient, and the viscosity of the refluxate. Viscosity is especially important in understanding why large volumes of gas can be vented from the stomach while at the same time largely containing fluid within the stomach; air has only 1/55th the viscosity of water allowing its flow rate to be 55-fold greater with all other variables constant.
Apart from selectively restricting liquid as opposed to gas reflux, the opening diameter of the EGJ is a primary determinant of the volume of gastric juice entering the esophagus during a reflux event. Even small increases in opening diameter result in an exponential increase in flow volume because flow rate is related to the opening radius to the fourth power. Furthermore, even patients without hiatus hernia may still have increased EGJ compliance secondary to more subtle defects not readily evident using current radiographic or endoscopic methods of evaluation. These subtle defects that likely dominate the pathophysiologic equation in some individuals may be more akin to minor anatomic features of the EGJ, such as an abnormal gastroesophageal flap valve, defects in the LES musculature, or a patulous diaphragmatic hiatus.
Lower esophageal sphincter
The LES is a 3- to 4-cm segment of tonically contracted smooth muscle at the EGJ. Anatomically, the LES is normally surrounded by the crural diaphragm, with greatest intraluminal pressure toward its proximal limit. In the setting of a sliding hiatus hernia, the LES is proximal to the crural diaphragm. Resting LES tone varies among normal individuals from 10 to 30 mm Hg relative to intragastric pressure and continuous pressure monitoring reveals considerable temporal variation. Large fluctuations of LES pressure occur with the migrating motor complex; during phase III, LES pressure may exceed 80 mm Hg. Lesser fluctuations occur throughout the day with pressure decreasing in the postcibal state and increasing during sleep. The genesis of LES tone is a property of both the smooth muscle itself and of its extrinsic innervation. At any given moment, LES pressure is affected by myogenic factors, intra-abdominal pressure, gastric distention, peptides, hormones, various foods, and many medications ( Table 1 ).
| Increase Lower Esophageal Sphincter Pressure | Decrease Lower Esophageal Sphincter Pressure | Increase Transient Lower Esophageal Sphincter Relaxations | Decrease Transient Lower Esophageal Sphincter Relaxations | |
|---|---|---|---|---|
| Foods | Protein |
| Fat | |
| Hormones |
|
| Cholecystokinin | |
| Neural agents |
|
| l -arginine | L-NAME Serotonin |
| Medications |
|
| Sumatriptan |
|
Crural diaphragm and hiatal canal
Surrounding the LES at the level of the SCJ is the crural diaphragm, most commonly the right limb of the right diaphragmatic crus arising from the upper lumbar vertebra. Two flattened muscle bundles of crural muscle split and incline forward to arch around the esophagus, first diverging like a scissor and then merging anteriorly with about a centimeter of muscle separating the anterior rim of the hiatus from the central tendon of the diaphragm. The hiatus is a teardrop-shaped canal about 2 cm along its major axis. The crural diaphragm is independently controlled during esophageal distention, vomiting, and belching when electrical activity is selectively inhibited regardless of continued respiration and costal diaphragm contraction. This anatomic and physiologic relationship is the basis for the “two sphincter hypothesis” for maintenance of EGJ competence, suggesting that both the intrinsic smooth muscle LES and the extrinsic crural diaphragm serve a sphincteric function.
Evidence suggesting a role of crural diaphragm dysfunction in GERD came from a recent study analyzing the pressure morphology and degree of inspiratory augmentation at the EGJ using high-resolution manometry in 156 GERD patients and 75 asymptomatic controls. EGJ pressure morphology was classified based on the degree of separation between the LES and crural contraction and the position of the pressure inversion point ( Fig. 1 ). The results of this analysis revealed that type III morphology was extremely rare in asymptomatic controls and patients with a negative endoscopy and a normal ambulatory pH study (<3%). In contrast, 30% of GERD patients with either a positive endoscopy or pH study had type III morphology.
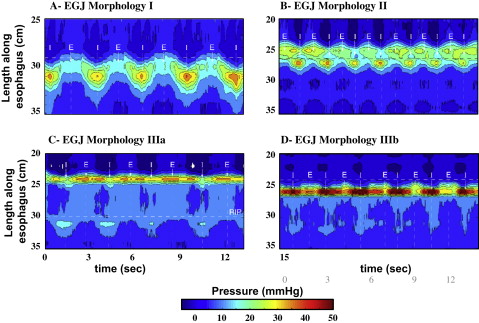
Inspiratory augmentation of EGJ pressure was significantly reduced in GERD patients, who were defined either by abnormal esophageal acid exposure during pH monitoring or a history of esophagitis, when compared with normal controls and symptomatic patients without objective evidence of GERD on endoscopy or ambulatory pH monitoring ( Fig. 2 ). Although both LES pressure and its separation from the pressure generated by the crural diaphragm were statistically correlated with GERD, the strongest association, and the only independent predictor of GERD as a categorical outcome in a logistic regression analysis, was impaired crural diaphragm dysfunction as indicated by reduced inspiratory augmentation of EGJ pressure. These data support the concept that the crural diaphragm functions as an extrinsic sphincter augmenting the competency of the antireflux barrier.
Gastroesophageal flap valve
In addition to the two sphincters described previously, another mechanism of barrier function at the EGJ lies in the positioning of the distal esophagus in the intra-abdominal cavity. With subdiaphragmatic positioning of the distal esophagus, a flap valve is formed by a musculomucosal fold created by the entry of the esophagus into the stomach along the lesser curvature. With this anatomic arrangement, increased intra-abdominal or intragastric pressure decreases the angle of His and compresses the subdiaphragmatic portion of the esophagus, thereby preventing opening and reflux during periods of abdominal straining. Although the clinical relevance of this concept remains controversial, several lines of evidence speak to its validity. Using cadavers without a hiatal hernia, Hill and colleagues demonstrated that surgically accentuating the length of the flap valve prevented reflux despite the presence of a gastroesophageal pressure gradient conducive to reflux attributable to increased intra-abdominal pressure. Hill and colleagues expanded on this concept with the development of a grading scheme of EGJ competence based on the endoscopic appearance of the gastroesophageal flap valve during retroflexion ( Fig. 3 ). Two endoscopic studies have reported that this grading scheme correlated with the severity of reflux disease. More recently, an investigation using wireless pH monitoring found a strong correlation between the degree to which individuals are susceptible to exercise-induced reflux and flap valve grade. No such correlation existed with LES pressure. Because exercise-induced reflux is presumably mediated by strain-induced increases in intra-abdominal pressure, these observations support the importance of the flap valve as a mechanism of EGJ competence.
Mechanisms of reflux
Prolonged manometric studies, done either bedside in a postprandial setting or using ambulatory manometry equipment, have facilitated the mechanistic analysis of how gastroesophageal reflux occurs. These investigations have come to focus on three dominant reflux mechanisms: (1) transient LES relaxations, without any necessary anatomic abnormality; (2) LES hypotension, again without necessarily invoking any anatomic abnormality; or (3) anatomic distortion of the EGJ inclusive of, but not limited to, hiatus hernia. Transient LES relaxations are essentially the only mechanism of reflux during periods of normal LES pressure (>10 mm Hg). They occur independently of swallowing; are not accompanied by peristalsis; are associated with crural diaphragm inhibition; are associated with distal esophageal shortening attributable to longitudinal muscle contraction; and persist for longer periods than swallow-induced LES relaxations (>10 seconds). Of note, prolonged manometric recordings have not consistently demonstrated an increased frequency of transient LES relaxations in GERD patients compared with normal controls; however, the frequency of acid reflux, as opposed to gas reflux, during transient LES relaxations has been consistently reported to be greater in GERD patients.
GERD can also occur in the context of diminished LES pressure either by strain-induced or free reflux. Strain-induced reflux occurs when a hypotensive LES is overcome and “blown open” in association with an abrupt increase of intra-abdominal pressure. Manometric data suggest that this rarely occurs when the LES pressure is greater than 10 mm Hg. It is also a rare occurrence in patients without hiatus hernia. Free reflux is characterized by a fall in intraesophageal pH without an identifiable change in either intragastric pressure or LES pressure. Episodes of free reflux are observed only when the LES pressure is within 0 to 4 mm Hg of intragastric pressure. A wide-open or patulous hiatus predisposes to this free reflux because both the intrinsic and extrinsic sphincters are compromised.
With hiatus hernia, crural diaphragm function is potentially compromised by both its axial displacement and atrophy consequent from dilatation of the hiatus. Another effect that hiatus hernia exerts on the antireflux barrier is to diminish the intraluminal pressure within the EGJ. Topographic representation of manometrically detectable EGJ high-pressure zone in hiatus hernia patients revealed distinct intrinsic sphincter and hiatal canal pressure components, with each one exerting pressure of lower magnitude than the EGJ pressure of a comparator group of normal controls. Simulating reduction of the hernia by repositioning the intrinsic sphincter back within the hiatal canal and arithmetically summing superimposed pressures resulted in calculated EGJ pressures, however, which were practically indistinguishable from those of the control subjects. Along with previous investigations these data demonstrated that hiatus hernia reduced the length of the EGJ high-pressure zone. This is likely caused by disruption of the EGJ distal to the squamocolumnar junction, the segment attributable to the opposing sling and clasp fibers of the gastric cardia. The effect of hiatus hernia on EGJ pressure morphology is also the likely explanation for the clinical observation that EGJ competence is inversely related to manometrically defined EGJ length put forth in a multitude of surgical publications.
Esophageal Acid Clearance
After an acid reflux event occurs, the duration of time that the esophageal mucosa remains acidified (pH <4) is termed the “esophageal acid clearance time.” Acid clearance begins as primary or secondary peristalsis empties the refluxed fluid from the esophagus. Esophageal pH is rarely restored to normal, however, by the first peristaltic sequence. Rather, this is achieved by subsequent swallows during which bicarbonate within swallowed saliva titrates the residual acid to normality. Prolongation of esophageal acid clearance among patients with esophagitis was demonstrated along with the initial description of an acid clearance test. Clinical data also suggest that prolonged acid clearance correlates with both the severity of esophagitis and the presence of Barrett’s metaplasia. From what is known regarding the mechanisms of acid clearance, the two main potential causes of prolonged esophageal acid clearance are impaired esophageal emptying and impaired salivary function.
Impairments of esophageal emptying
Impaired esophageal emptying in GERD was inferred by the observation that symptoms of gastroesophageal reflux improve with an upright posture, a maneuver that allows gravity to augment fluid emptying. Subsequently, two mechanisms of impaired esophageal emptying have been identified: peristaltic dysfunction and superimposed reflux associated with nonreducing hiatus hernias. Peristaltic dysfunction in esophagitis has been described by a number of investigators. Of particular significance are failed peristalsis and hypotensive peristaltic contractions (<30 mm Hg), which result in incomplete emptying. As esophagitis increases in severity, so does the incidence of peristaltic dysfunction, and recent studies show no peristaltic improvement after healing of esophagitis by acid inhibition, or by antireflux surgery. Most likely, the acute dysfunction associated with active esophagitis is partially reversible, but not that associated with strictures or fibrosis.
Hiatus hernia can also impair esophageal emptying. Concurrent pH recording and scintigraphy above the EGJ showed that impaired esophageal clearance was caused by reflux of fluid from the hernia sac during swallowing. This observation was subsequently confirmed radiographically in an analysis of esophageal emptying in patients with reducing and nonreducing hiatus hernias. The efficacy of emptying was significantly diminished in both hernia groups when compared with normal controls. Emptying was particularly impaired in the nonreducing hiatus hernia patients who exhibited complete emptying with only one third of test swallows. Consistent with the scintigraphic studies, the patients with nonreducing hernias were the only group that exhibited retrograde flow of fluid from the hernia during deglutitive relaxation.
Salivary function
The final phase of esophageal acid clearance is facilitated by salivation. Just as impaired esophageal emptying prolongs acid clearance, diminished capacity for the saliva to neutralize any remaining intraesophageal acid has a similar effect. Diminished salivation during sleep, for instance, explains why reflux events during sleep or immediately before sleep are associated with markedly prolonged acid clearance times. Chronic xerostomia is also associated with prolonged esophageal acid exposure and esophagitis. One group of subjects shown to have prolonged esophageal acid clearance times attributable to hyposalivation is cigarette smokers. Even those without symptoms of reflux disease exhibited acid clearance times 50% longer than those of nonsmokers and the salivary titratable base content was only 60% of the age-matched nonsmokers. No systematic difference has been found, however, in the salivary function of GERD patients when compared with controls.
In addition to bicarbonate, saliva contains growth factors that have the potential to enhance mucosal repair. Epidermal growth factor, produced in submaxillary ductal cells and duodenal Brunner’s glands, has been extensively studied. In animal models, epidermal growth factor has been shown to provide cytoprotection against irritants, enhance the healing of gastroduodenal ulceration, and decrease the permeability of the esophageal mucosa to hydrogen ions. Studies have not shown consistent differences in epidermal growth factor concentration in esophagitis or Barrett’s metaplasia patients, however, making it impossible to implicate perturbations of growth factor secretion in GERD pathogenesis.
Causticity of the Gastric Refluxate
Gastric juice contains substances that can be particularly harmful to the esophageal epithelium with prolonged exposure. Gastric (hydrochloric) acid is the most noxious component and continues to be the initial therapy target for medications that either neutralize it or inhibit its secretion. Severe erosive esophagitis occurs in 42% to 70% of patients with Zollinger-Ellison syndrome; excessive acid production seems an attractive causative factor in GERD pathogenesis. Although there have been reports of greater rates of gastric acid secretion in patients with reflux esophagitis, a prospective study in a large population using gender-matched controls showed that basal acid output in male patients with esophagitis is similar to those without (3.8 ± 0.5 versus 4 ± 0.4 mEq/h). The output seen in female patients with esophagitis only seems to be increased when contrasted with the low output in females with no esophagitis (3.4 ± 0.6 versus 1.7 ± 0.2 mEq/h), but more importantly, no significant correlation to the grade of the esophagitis was seen in either gender esophagitis group. At the other end of the spectrum, H pylori may play a protective role against development of esophagitis by inducing atrophic gastritis with concomitant hypoacidity. Furthermore, patients with previous peptic ulcer disease who had their H pylori eradicated may develop GERD at a later stage. This relationship is tenuous, however, considering reports indicating no differences in any reflux variables between H pylori –positive and –negative patients and that the prevalence of this bacterium is not greater in esophagitis patients than in controls.
Pepsin, bile, and pancreatic enzymes within gastric secretions can injure the esophageal epithelium, but their noxiousness is either lessened in an acidic environment, dependant on acidity for activation, or limited by the observation that the concentrations found physiologically are too low to be clinically relevant. Bile warrants some attention because it is the second predominant component of refluxate and it persists in refluxate despite acid-suppressing medications. Bile can transverse the cell membrane imparting severe cellular injury in a weakly acidic environment (pH 3–5) that may exist on acid-suppressing therapy. For example, in a rat reflux model, surgically induced bile reflux in a mildly acidic environment increased esophageal columnar metaplasia and the development of adenocarcinoma. Hence, the causticity of gastric refluxate extends beyond the exposure to gastric acid.
Pathophysiology of gastroesophageal reflux disease
Gastroesophageal reflux is a normal physiologic event, but excessive exposure of esophageal or supraesophageal epithelium to gastric refluxate resulting in either mucosal injury or related symptoms is the fundamental abnormality in GERD. The genesis of reflux injury and reflux symptoms are not the same but each relates to some combination on pathophysiologic factors leading to an excessive number of gastroesophageal reflux events, impaired clearance of refluxate from the esophagus, increased acidity of the refluxed gastric juice, or increased sensitivity of the esophageal or supraesophageal mucosa to that refluxate. Aberrations in one or more potential pathophysiologic factors can result in creation of conditions conducive to the development of esophagitis or such symptoms as heartburn. This shift from a compensated to a decompensated condition can be conceptualized as a dynamic balance between the aggressive forces promoting reflux, mucosal injury, and symptom generation and defensive forces that counteract these.
Antireflux Barrier
The primary line of defense against GERD is the integrity of the antireflux barrier. GERD, and all that it entails, cannot occur unless the antireflux barrier permits gastric juice to enter the esophagus. Localized at the esophagogastric junction (EGJ), the antireflux barrier is an anatomically complex zone whose functional integrity in preventing reflux has been variably attributed to intrinsic lower esophageal sphincter (LES) pressure, extrinsic compression of the LES by the crural diaphragm, the intra-abdominal location of the LES, integrity of the phrenoesophageal ligament, and maintenance of the acute angle of His promoting a “flap valve” function. The global function of the EGJ as an antireflux barrier is dependent on the sum of its constituent parts and their ability to maintain a high-pressure zone or a closed luminal segment in the region separating the stomach from the esophagus. The EGJ high-pressure zone is typically maintained during the interdeglutitive state by contraction of the LES and surrounding crural diaphragm.
An important distinction to make with respect to reflux is between EGJ relaxation, evident by the absence of a contractile pressure, and EGJ opening, wherein an intraluminal space is created. Relaxation is not always associated with opening. Furthermore, even with opening, the volume of ensuing reflux is strongly related to the dimensions of luminal opening that occurs. The volume of reflux that occurs following opening is strongly related to the opening dimensions of the EGJ, the pressure gradient between the gastroesophageal pressure gradient, and the viscosity of the refluxate. Viscosity is especially important in understanding why large volumes of gas can be vented from the stomach while at the same time largely containing fluid within the stomach; air has only 1/55th the viscosity of water allowing its flow rate to be 55-fold greater with all other variables constant.
Apart from selectively restricting liquid as opposed to gas reflux, the opening diameter of the EGJ is a primary determinant of the volume of gastric juice entering the esophagus during a reflux event. Even small increases in opening diameter result in an exponential increase in flow volume because flow rate is related to the opening radius to the fourth power. Furthermore, even patients without hiatus hernia may still have increased EGJ compliance secondary to more subtle defects not readily evident using current radiographic or endoscopic methods of evaluation. These subtle defects that likely dominate the pathophysiologic equation in some individuals may be more akin to minor anatomic features of the EGJ, such as an abnormal gastroesophageal flap valve, defects in the LES musculature, or a patulous diaphragmatic hiatus.
Lower esophageal sphincter
The LES is a 3- to 4-cm segment of tonically contracted smooth muscle at the EGJ. Anatomically, the LES is normally surrounded by the crural diaphragm, with greatest intraluminal pressure toward its proximal limit. In the setting of a sliding hiatus hernia, the LES is proximal to the crural diaphragm. Resting LES tone varies among normal individuals from 10 to 30 mm Hg relative to intragastric pressure and continuous pressure monitoring reveals considerable temporal variation. Large fluctuations of LES pressure occur with the migrating motor complex; during phase III, LES pressure may exceed 80 mm Hg. Lesser fluctuations occur throughout the day with pressure decreasing in the postcibal state and increasing during sleep. The genesis of LES tone is a property of both the smooth muscle itself and of its extrinsic innervation. At any given moment, LES pressure is affected by myogenic factors, intra-abdominal pressure, gastric distention, peptides, hormones, various foods, and many medications ( Table 1 ).






