Fig. 7.1
Traditional laparoscopic anti-GERD therapies. GERD gastroesophageal reflux disease
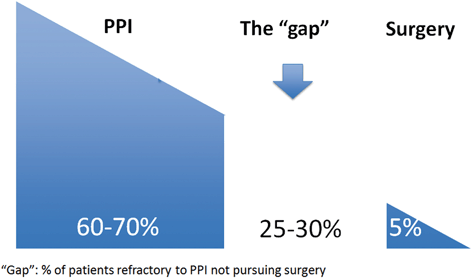
Fig. 7.2
Treatment gap in the management of GERD. PPI proton pump inhibitors. (Reprinted from Ref. [9])
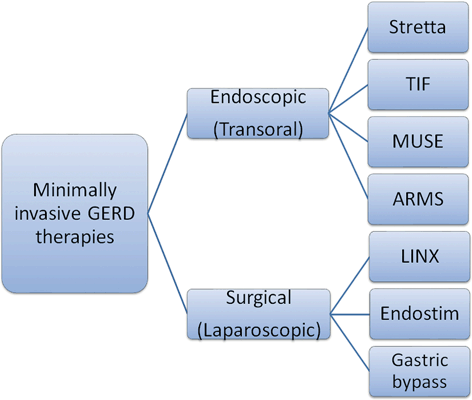
Fig. 7.3
Outline of the various minimally invasive, transoral, and laparoscopic anti-GERD therapies. GERD gastroesophageal reflux disease, TIF transoral incisionless fundoplication, MUSE Medigus Ultrasonic Surgical Endostapler, ARMS anti-reflux mucosectomy
The advantage of these procedures is that they do not dramatically alter the anatomy of the esophagogastric junction (EGJ), esophagus, or stomach, and thus they have a better side-effect profile. By design, these techniques are intended to target patients with mild EGJ defects, and, thus, they should not be considered as alternatives to fundoplication and hernia repair for patients with significant anatomic abnormalities [4]. Table 7.1 outlines the most common reasons to consider minimally invasive GERD therapies.
Table 7.1
Reasons to consider minimally invasive GERD therapies
Refractory acid reflux and esophagitis despite high-dose PPI |
Intolerance to PPI |
Inability to comply with daily PPI |
Concern about potential PPI-induced long-term adverse effects |
Concern about potential short- and long-term adverse effects of surgical fundoplication |
Costs of long-term PPI |
Before considering if a minimally invasive GERD therapy is appropriate for a particular patient, it is essential that the diagnosis of GERD is established and other confounding factors have been excluded [5]. Furthermore, there should be confidence that the presenting GERD symptoms are truly reflective of GERD and that the proposed therapy has the potential to eliminate or significantly reduce them. A careful review of the possible determinants of GERD for each patient is essential, since it will help highlight the best strategy (Fig. 7.4) [6]. Unfortunately, various outcome measures have been used to assess the efficacy of minimally invasive GERD therapies, and frequently the lack of efficacy on one or more of these measures is used as a deterring element in the decision-making process. Table 7.2 highlights the most frequent outcome measures used in clinical trials. It is important to note that all of these measures have limitations and that any decision has to be individualized to the particular patient and their expectations. For example, complete normalization of esophageal acid exposure time may not be important in a patient who has refractory heartburn despite PPI use, as long as the symptoms improve with the intervention [4]. Similarly, a patient who manages to eliminate volume reflux (regurgitation) but continues to use PPI after a minimally invasive GERD therapy should not be considered a treatment failure. Elimination of troublesome regurgitation and healing of esophagitis are robust clinical end points, but they have only recently been examined in clinical trials [7].
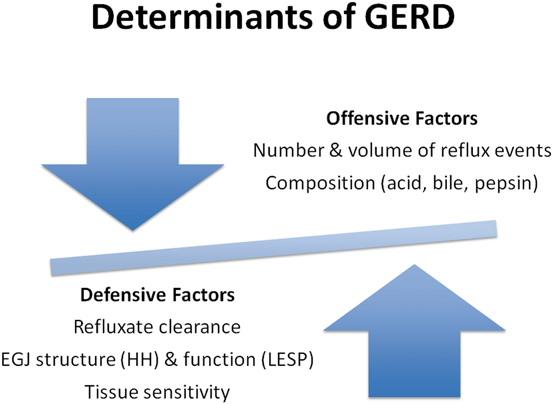

Fig. 7.4
Determinants of GERD that need to be considered prior to an individualized endoscopic or laparoscopic intervention. EGJ esophagogastric junction, HH hiatal hernia, LESP lower esophageal sphincter pressure
Table 7.2
Outcome measures of the efficacy of minimally invasive GERD therapies
Healing of esophagitis |
Symptoms (heartburn, regurgitation, etc.) |
GERD-related quality of life |
PPI use |
Esophageal ambulatory pH and impedance testing |
Esophageal manometry |
The suitability of a patient for minimally invasive GERD therapy also depends on a careful assessment, both structural and functional, of the EGJ and other factors that could aggravate or precipitate GERD symptoms. Table 7.3 highlights the key determinants of EGJ competence that require expert assessment by endoscopy, high-resolution manometry, and barium swallow [8]. Other elements such as the effectiveness of esophageal peristalsis, gastric emptying rate, body mass index (BMI), or prior esophagogastric surgery will also need consideration. The probability of success or failure for each minimally invasive GERD therapy varies significantly, depending on the structural and functional characteristics of an individual patient who has been carefully evaluated and appropriately selected. Clinical trials to date have included a mixed group of patients, resulting in variable and, at times, unsatisfactory results [9]. Table 7.4 outlines the essentials of patient assessment prior to proceeding with anyone of the available minimally invasive endoscopic or surgical techniques.
Table 7.3
Determinants of EGJ competence
Intrinsic LES pressure |
Intra-abdominal location of the LES |
Extrinsic compression of the LES by the crural diaphragm |
Integrity of the phreno-esophageal ligament |
Preservation of the acute angle of His |
Table 7.4
Essentials of patient assessment
History and physical examination (including BMI) |
Prior history of esophagogastric surgery |
Endoscopy with biopsies |
Endoscopic and radiological assessment of the EGJ |
High-resolution manometry (definition of hiatal hernia and peristaltic effectiveness |
Esophageal pH/impedance monitoring and symptom association |
Gastric emptying |
Figure 7.3 outlines the available minimally invasive GERD therapies that need to be entertained in selected patients. Many of these therapies are at an early stage of their development and utility, and some of them are still not approved in the USA. Their strengths and weaknesses, suitability, and effectiveness need to be balanced against the traditional laparoscopic approaches (with or without hernia repair) or continuation of pharmacologic therapy and lifestyle measures.
Minimally Invasive Endoscopic (Transoral) Therapies
Since the early 2000s, several devices have been developed for the endoscopic treatment of GERD, using approaches such as sewing, transmural fasteners , endoscopic staplers, and thermal treatment using radio-frequency energy. Other devices involving injection (Enteryx, Boston Scientific, Boston, MA, USA) or implantation of foreign materials (Gatekeeper Reflux Repair System, Medtronic, Inc., Minneapolis, MN, USA) at the esophageal junction have been withdrawn from the market. Devices that are currently commercially available for the endoscopic treatment of GERD in the USA include the following: Stretta (Mederi Therapeutics, Greenwich, CT, USA), transoral fundoplication (TF, EndoGastric Solutions, Redmond, WA, USA) , and the Medigus Ultrasonic Surgical Endostapler (MUSE™) system (previously known as Supplemental Restraint System (SRS™) system for TF; Medigus, Omer, Israel). Anti-reflux mucosectomy (ARMS) has been recently described, but it is not yet approved for the management of GERD.
Stretta
The Device
Stretta comprises a four-channel radio-frequency (RF) generator and a four-needle balloon–catheter system that delivers pure sine-wave energy (465 kHz, 2–5 W per channel, 80 V maximum at 100–800 Ω). Each needle tip incorporates a thermocouple that automatically adjusts the power output to a desired target temperature of 85 °C in the muscle layer. Temperature is similarly monitored with a thermocouple at each needle base abutting the mucosa, and the power delivery ceases if such mucosal temperature exceeds 50 °C or if impedance exceeds 1000 mΩ. Maintaining tight temperature control prevents mucosal damage, thus preventing stricture formation [10]. The Food and Drug Administration (FDA) originally cleared Stretta for use in 2000 and issued an updated clearance on the RF1 generator in 2011.
The Procedure
An upper gastrointestinal (GI) endoscopy is first performed and the distance from the incisors to the squamocolumnar junction (Z line) is measured. The endoscope is removed, and the RF catheter is passed through the mouth and positioned 1 cm above the Z line according to the distance previously determined. The four needle electrodes are deployed to a preset length of 5.5 mm, and RF delivery is initiated. Additional applications, by rotating and changing the linear position of the catheter, create several rings over a span of 2 cm above and below cardia. The catheter is then removed, and the endoscopy is repeated. Overall, patients receive RF energy at 56 treatment sites over a period of 35 min (Fig. 7.5). Although the exact mechanism of action of Stretta in relieving symptoms of acid reflux is unknown, one potential mechanism is that it decreases the number of transient lower esophageal sphincter relaxations (TLESRs) through a structural rearrangement of the smooth muscle and redistribution of the interstitial cells of Cajal in the smooth muscle of the lower esophageal sphincter (LES) [11].
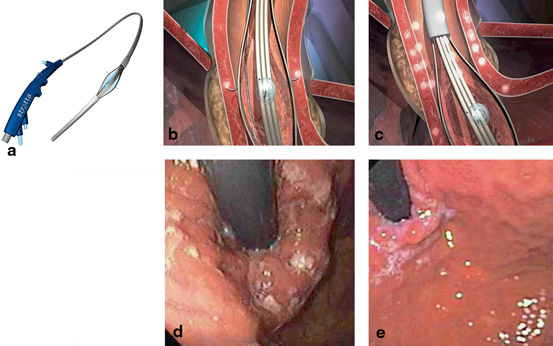

Fig. 7.5
a The Stretta catheter. b, c Diagram of the Stretta procedure depicting the balloon-needle assembly that delivers RF energy to the muscle of the EGJ region (Courtesy of Mederi Inc.). d Retroflexed endoscopic image of the cardia immediately after RF energy delivery. e Retroflexed endoscopic image of the cardia 3 months after Stretta. (Courtesy of George Triadafilopoulos, MD)
The Data
Multiple studies, including four randomized clinical trials, have demonstrated the safety and efficacy of Stretta for GERD therapy, and a high rate of symptom control and decrease or elimination of GERD medication use have been consistently achieved. As the endoscopic procedure with the most available data and track record, Stretta appears to be safe, effective, durable, and repeatable, if necessary. Several putative mechanisms could explain Stretta’s clinical effectiveness, and they include increased gastric yield pressure, increased thickness of the LES muscle, decreased distensibility of the EGJ without fibrosis, decreased EGJ compliance, and decreased frequency of TLESRs . A recent double-blind sham-controlled study of 22 patients showed that the administration of sildenafil, an esophageal smooth muscle relaxant, normalized the EGJ compliance to pre-Stretta levels, arguing against EGJ fibrosis as an underlying mechanism. Two cohort studies found no adverse effects on vagal function and no significant changes in esophageal motility or swallow-induced LES relaxation pressure arguing against a neurolytic effect. Initial animal studies used porcine and canine models and showed a thickening of the LES, decreased TLESRs, and decreased reflux events [11].
A randomized, sham-controlled trial assigned 64 GERD patients to Stretta or to a sham procedure [12]. At 6 months, active treatment significantly improved patients’ heartburn symptoms and quality of life. More active versus sham patients were without daily heartburn symptoms (61 vs. 33 %; p = 0.05), and more had a > 50 % improvement in their GERD-health-related quality-of-life (HRQL) scores (61 vs. 30 %; p = 0.03). Another randomized prospective trial included 36 patients who were randomized into three groups: single-session Stretta, sham procedure, and single Stretta followed by repeat Stretta if GERD-HRQL was not 75 % improved after 4 months [13]. At 12 months, the mean HRQL scores of those “off” medications, the LES basal pressure, the 24-h pH scores, and the PPI daily dose consumption were significantly improved from baseline in both Stretta groups (p < 0.01). Seven patients in the double Stretta treatment group had normalized their HRQL at 12 months compared with two patients in the single-treatment group (p = 0.035). Like the other newer techniques, Stretta has not been found useful in patients with hiatal hernias > 3 cm, those with no previous response to PPIs, and those with negative pH or impedance studies.
A recent meta-analysis of 18 studies and 1488 patients concluded that Stretta (1) is very effective in GERD symptom relief, (2) is safe and well tolerated, and (3) significantly reduces acid exposure to the esophagus, but does not consistently normalize pH [14]. On this last point, it is important to note that even PPIs do not normalize pH in up to 50 % of symptomatically controlled GERD patients treated with PPIs. Hence, pH normalization is not necessarily an essential clinical end point to be applied to Stretta. In a single-center, long-term (10 years) study, normalization of GERD-related quality of life was achieved in 72 % of patients; a 50 % or greater reduction in PPI use occurred in 64 % of patients (41 % eliminating PPIs entirely), and a 60 % or greater increase in satisfaction occurred in 54 % of patients. Preexisting Barrett’s metaplasia regressed in 85 % of biopsied patients. Another meta-analysis, however, found that Stretta compared with sham therapy for patients with GERD does not produce significant changes, in physiologic parameters, including time spent at a pH less than 4, LES pressure, ability to stop PPIs, or HRQL [15].
Limitations
Stretta is not to be used in patients with sliding hiatal hernia (> 2 cm), severe (> Los Angeles (LA) grade B) esophagitis, or Barrett’s esophagus. Data on the procedure’s effectiveness and durability have at times produced mixed results. Definitive conclusions have been problematic because of the heterogeneity of measured variables in different studies of variable patient populations.
Summary
With several randomized sham-controlled trials and more than 40 short- and long-term studies, Stretta is a safe, effective, and mature technology and repeatable if necessary [16, 17]. Further, it is the least expensive alternative to medical therapy, and it does not preclude the subsequent use of any other alternative therapy for GERD.
TF
The Device
The device creates molding of tissue and placement of polypropylene suture material in the region of the EGJ. It is composed of a controls handle , a chassis through which the endoscope is inserted and control channels run, side holes on the distal end of the shaft to which external suction can be applied, a tissue mold that pushes tissue against the shaft of the device, a helical screw, which is advanced into tissue to pull tissue, two stylets, which advance from the shaft of the device through the plicated tissue and then through eyelets in the tissue mold, and a cartridge containing polypropylene H-shaped fasteners [18].
The Procedure
TF is a newer technique devised to perform a partial fundoplication endoscopically (Fig. 7.6). The device retracts the gastric cardia and creates full-thickness serosa-to-serosa plication and valve. In contrast to surgical fundoplication, TF does not involve any abdominal incisions or dissections that could increase the risk for adhesions and complications and is associated with less discomfort and faster recovery. TF has been found to reduce the number of postprandial TLESRs, the number of TLESRs associated with reflux, and EGJ distensibility, leading to a reduction of the number and proximal extent of reflux episodes and improvement of acid exposure [18].
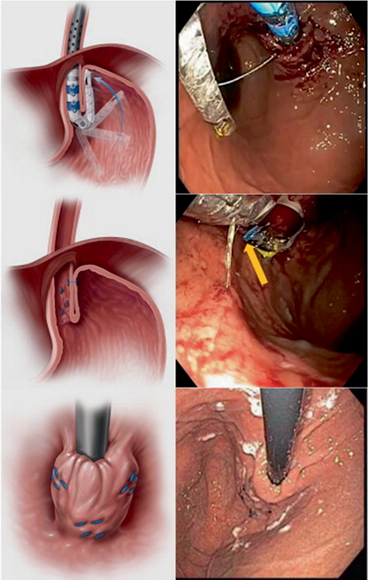

Fig. 7.6
Transoral fundoplication that has created a 3-cm flap valve, 180°–270° in circumference. The valve was created with a minimum of 13 fasteners and was at least 1 cm long at either corner, and 3 cm long in its mid-portion. (Reprinted with permission from [28] )
The Data
Several studies have asserted the efficacy and safety of TF. A prospective, sham-controlled trial aimed to determine whether TF would reduce regurgitation more than PPI therapy in patients with GERD without significant (> 2 cm) hiatal hernia [19]. Patients were randomly assigned to groups that underwent TF and then received 6 months of placebo (n = 87), or sham procedure and 6 months of once- or twice-daily omeprazole (controls, n = 42). Patients were blinded to therapy and reassessed at 2, 12, and 26 weeks. By intention-to-treat analysis, TF eliminated regurgitation in 67 % of patients, more than with omeprazole (45 %; p = 0.023). Esophageal acid exposure improved but did not normalize after TF (mean 9.3 % before and 6.3 % after; p < 0.001), but not after sham procedure (mean 8.6 % before and 8.9 % after). Subjects from both groups who completed the protocol had similar reductions in GERD symptom scores. Severe complications were rare (three subjects receiving TF and one receiving the sham procedure).
In patients with incomplete symptom control on high-dose PPI therapy, TF may provide further elimination of symptoms and heal esophagitis [20]. A randomized, multicenter, open-label, crossover study aimed to evaluate if TF could further improve clinical outcomes in partial responders to high-dose PPI therapy and to evaluate the durability of such effect. Patients with GERD and hiatal hernia ≤ 2 cm were randomized to TF (n = 40) or high-dose PPI therapy (n = 23) group. At 6-month follow-up, PPI patients underwent crossover. The investigators then assessed clinical outcomes 6 months post TF in crossover patients, as compared to 6 months of PPI therapy, and 12-month outcomes in patients initially randomized to TF. The primary outcome was symptom control using standard questionnaires. There were 39 analyzable TF patients and 21 crossover patients. In the latter group, TF further improved control of regurgitation and of atypical symptoms achieved after 6 months of PPI. Of 20 patients with GERD symptoms after 6 months of high-dose PPI therapy, 65 % (13/20) reported global elimination of troublesome regurgitation and atypical symptoms post TF off PPI; 67 % (6/9) reported no significant regurgitation. Esophagitis further healed in 75 % (6/8) of patients. Seventy-one percent of crossover patients were off PPI 6 months following TF. In the original TF group, 12-month post-TF, 77 % of patients achieved complete symptom control, 82 % ceased PPI therapy, 100 % healed esophagitis, and 45 % normalized esophageal acid exposure.
An open, prospective, multicenter study assessed the 2-year symptom control of TF [21]. Secondary outcomes were PPI use, degree of esophagitis , safety, and changes in esophageal acid exposure. Of the 127 patients who underwent TF, 15 % were lost to follow-up; 8 patients underwent revisional surgery but were included, as failures. No serious adverse events were reported. Scores for GERD-related HRQL and regurgitation improved by > 50 % in 66 and 70 % of patients, respectively. Reflux scores normalized in 65 % of patients, and daily PPI use decreased from 91 to 29 %.
Another open, single-center study assessed the long-term effect of TF on pathological reflux and symptoms in 50 GERD patients who were dependent on PPI therapy and found that TF achieved lasting elimination of daily dependence on PPI in 75–80 % of patients for up to 6 years [22]. In all, 83.7, 79.6, 87.8, and 84.4 % of patients stopped or halved the PPI therapy 6, 12, 24, and 36 months after TF. Impedance monitoring indicated significantly fewer total and acid refluxes after treatment (p = 0.01). Factors predicting good outcomes were pre-procedure Hill’s grade I–II, no hiatal hernia or hernia ≤ 2 cm (p = 0.03), the absence of ineffective esophageal motility (p < 0.0001), and the number of fasteners deployed (p = 0.01). In patients who fail TF, LARS is a feasible and safe option without additional operative morbidity [23].
Limitations
The available data are limited to patients without significant hiatal hernia (< 2 cm).
Summary
Transoral incisionless fundoplication (TIF) has emerged as a safe, effective, and durable alternative to GERD patients who do not respond completely to PPI without the adverse event profile associated with LARS.
MUSE™
The Device
The MUSE™ endoscopic stapling system is a recently introduced technique capable of creating an endoscopic partial fundoplication . The device consists of a flexible endoscope, a video camera, an ultrasonic range finder, and a surgical stapler.
The Procedure
The MUSE endoscope is inserted and advanced into the stomach and retroflexed, pulling it back to the correct stapling level above the EGJ. Tissue is then clamped and stapled under ultrasonographic gap finder. The procedure is repeated a few times to form a flap, representing a 180° fundoplication (Fig. 7.7) [24].
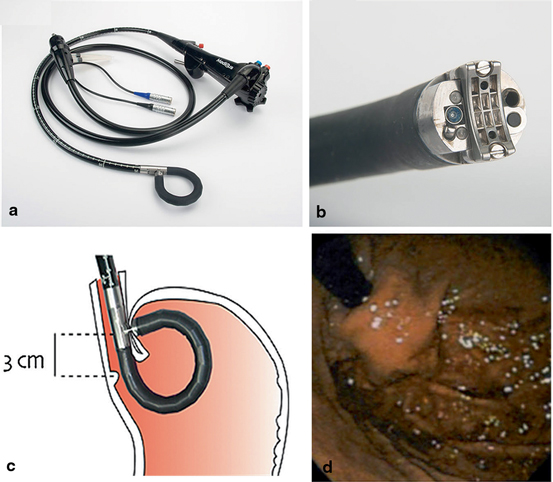

Fig. 7.7
Medigus transoral surgical stapler (MUSE™): a Full flexible endostapler, outside diameter (OD) 15.5 mm. b Distal tip. c Positioning of cartridge 3 cm proximal to gastroesophageal junction for stapling. d Gastric cardia (retroflexed) view of an effective gastroesophageal flap valve. (Reprinted with permission from Ref. [26] )
The Data
The procedure has shown promise in a preclinical trial, where 12 study animals underwent the procedure, and all of them had a satisfactory partial fundoplication , with no procedure-related complications [25]. One of the first human trials using an earlier version of MUSE was conducted to compare it with LARS. Of 27 non-randomized patients, 11 underwent MUSE and 16 underwent LARS. Over a 6-month follow-up, a decrease in GERD-HRQL scores was achieved in 64 and 87 % of patients who had MUSE and LARS, respectively. An esophageal perforation observed in the endoscopic group completely recovered after over-the-scope clipping. Procedure times for MUSE and LARS were 89 and 47 min, respectively (p < 0.05). During 6 months mean follow-up, PPI use was similar, and GERD-HRQL scores dropped in both groups.
A multicenter, prospective study evaluated the clinical experiences of 69 patients who received endoscopic anterior fundoplication with a video- and ultrasound-guided transoral surgical stapler [26]. Its initial 6-month data demonstrated safety and efficacy but necessitated procedure and device changes to improve safety, which in turn led to improved results in the later portion of the study. Of the 66 patients who completed follow-up 6 months after the procedure, the GERD-HRQL score improved by > 50 % off PPI in 73 % of patients and 64.6 % were no longer using daily PPI medication. Common adverse events were perioperative chest discomfort and sore throat. Two severe adverse events (empyema and GI bleeding) requiring intervention occurred in the first 24 subjects, but no further esophageal injury was noted in the remaining patients.
Limitations
Larger randomized studies with longer periods of follow-up are required before its clinical use is considered. Continued assessment of durability and safety are ongoing.
Summary
Very early experience with different versions of the device is promising, but inconclusive and randomized trials are needed.
ARMS
The Device
Very recently, Japanese authors have reported the clinical outcomes of two case series in which they used conventional endoscopic polypectomy and dissection tools to perform ARMS [27] .
The Procedure
ARMS is performed using endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) of at least 3 cm length (1 cm in the esophagus and 2 cm in the stomach), with the length of mucosal resection at the cardia measured in retroflexion from the gastric side. It is preferably performed in a crescentic fashion along the side of the lesser curve of the stomach, thus preserving a sharp mucosal valve at gastric cardia (Fig. 7.8) .
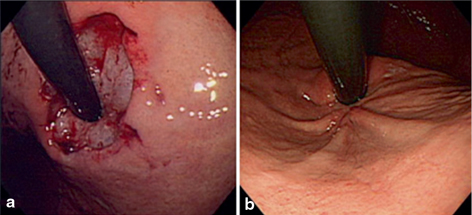





Fig. 7.8
Endoscopic follow-up of circumferential anti-reflux mucosectomy (ARMS) (retroflexed views). a Immediately after circumferential ARMS. Approximately 2 cm-wide gastric cardia mucosa was circumferentially resected by cap-endoscopic mucosal resection method. b Appearance at 3 years revealing a tight gastroesophageal junction with convergence of three gastric folds along the lesser curve of the stomach. (Reprinted with permission from Ref. [28])
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






