CHAPTER 2 Minimally invasive esophagectomy
Step 1. Surgical anatomy
♦ Adenocarcinoma is the most common type of esophageal cancer in Western society, and esophagectomy is the primary therapy for resectable tumors. Traditional “open” esophagectomy has been associated with significant morbidity and mortality rates. In an attempt to lower these rates, minimally invasive techniques were introduced. Two laparoscopic approaches, each indicated for different stages of disease, are described in this chapter.
♦ Laparoscopic transhiatal inversion esophagectomy (LIE) with gastric substitution can be employed for treatment of end-stage benign disease (Barrett’s high-grade dysplasia; achalasia) and early malignancy confined to the mucosa (T1a stage).
♦ Esophageal cancer is known for early and rapid dissemination because of the longitudinally oriented lymphatic plexus within the submucosa with direct transmural lymphatic connections and the lack of a serosal lining. Although lymph node involvement is infrequent in T1a-stage adenocarcinoma of the esophagus, lymph node involvement increases nearly 10-fold in T1b-stage (submucosal) disease.
♦ The combined laparoscopic-thoracoscopic (two-cavity) approach with en bloc lymph-adenectomy is indicated for treatment of resectable advanced locoregional disease.
Step 2. Preoperative considerations
Patient preparation
♦ Preoperative evaluation and staging includes endoscopy, bronchoscopy, endosonography and positron emission tomography combined with computed tomography (PET-CT) scanning.
♦ Preoperative evaluation of comorbid conditions should include at least an evaluation of a patient’s cardiopulmonary reserve. In selected cases with severe peripheral occlusive arterial disease, a visceral angiogram is obtained.
♦ A preoperative exercise program, smoking cessation, and optimization of nutritional status should be endeavored.
♦ Preoperative mechanical bowel preparation is performed when colon interposition may be required.
Equipment and instrumentation
♦ Blunt port (Covidien, Mansfield, Massachusetts) 5 to 12 mm
♦ 30-degree and 45-degree 10 mm endoscope and a 5-mm 30-degree endoscope
♦ Needle feeding jejunostomy kit (Compat Biosystems, Minneapolis, Minnesota)
♦ Autosonix ultrasonic scalpel (Covidien, Mansfield, Massachusetts)
♦ Diamond-flex liver retractor or Nathanson liver retractor for left lobe of liver
♦ Endoscopic retractor (10 mm) for retracting lung
♦ Large endoscopic clips applier
Anesthesia
♦ Prior to induction, a thoracic epidural is placed for postoperative pain control, and antibiotic prophylaxis (second-generation cephalosporin) is administered.
♦ Endotracheal intubation is performed with a single lumen tube in LIE. In combined laparoscopic-thoracoscopic (two-cavity)-approach, a double lumen endotracheal tube is required for single-lung ventilation.
♦ A nasogastric tube and a urinary catheter are placed, and an arterial catheter for continuous blood pressure monitoring is instituted.
Step 3. Operative steps
Laparoscopic transhiatal inversion esophagectomy
Patient positioning
♦ After induction, an intraoperative bronchoscopy and esophagogastroduodenoscopy are performed for assessment of anatomic relationships and tumor location.
♦ Skin preparation and draping of the abdomen, chest, and left side of the neck is performed in a single field.
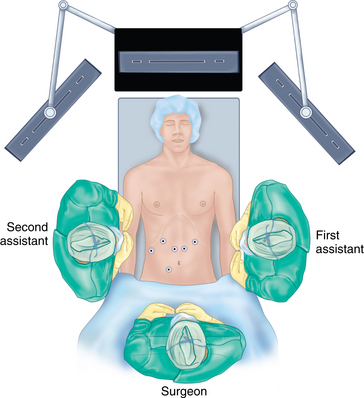
♦ For the abdominal portion of laparoscopic esophagectomy, the patient is placed in a supine, split-legged position and secured to the operation table with supportive padding for all pressure zones. A cushion can be placed at scapula level to induce slight neck extension for cervical exposure, if a neck anastomosis is planned.
♦ The surgeon stands between the patient’s legs (French position); the first assistant is positioned at the patient’s left and the second assistant at the patient’s right.
Port placement
♦ After pneumoperitoneum is obtained by using Veress needle technique, the primary site of access is approximately 15 cm below the left costal margin, 3 cm out of the midline. A 45-degree laparoscope is introduced through a 10-mm port and, before secondary port placements, a staging laparoscopy is performed.
♦ A six-port approach is used with the remaining ports in the following locations: second port (12 mm, surgeon’s right hand) 12 cm from the xiphoid process, 2 cm below the left costal margin; third port (5 mm, first assistant) left anterior axillary line along the costal margin; fourth port (5 mm, liver retractor) left of the xiphoid process; fifth port (12 mm, surgeon’s left hand, access endoscopic stapling device) inferior to the right costal margin and immediately to the right of the falciform ligament; sixth port (5 mm, second assistant) right midabdominal position, based on internal anatomy (Figure 2-1).
Hiatal dissection
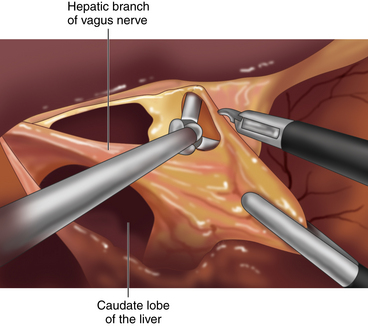
♦ Using a liver retractor, the hiatal opening and the gastrohepatic omentum are exposed and divided, along with the hepatic branch of the anterior vagus trunk, using the Harmonic scalpel (Figure 2-2).
♦ A replaced left hepatic artery in the lesser omentum, which arises from the left gastric artery in about 30% of population, should be spared.
♦ The phrenoesophageal membrane is opened around the esophagus until both the crural pillars are dissected and the esophagus can be encompassed.
♦ In LIE indicated for benign disease or early malignancies, the vagal nerves are preserved to maintain gastric physiology.
Gastric mobilization
♦ The epiphrenic fat is retracted toward the left anterior abdominal wall to provide tension on the left gastric vessels. The overlying peritoneum is opened, using the Harmonic scalpel, and the left gastric vessels are identified and divided using an endoscopic vascular stapler (Figure 2-3).
♦ The gastric fundus is mobilized with the division of the short vessels.
♦ The gastrocolic omentum is divided along the greater curvature. At Demel’s (watershed) point, the union of left and right gastroepiploic vessels is preserved to protect the blood supply to the gastric conduit (Figure 2-4).
♦ The gastrosplenic ligament is divided, including the intervening short gastric vessels, and the gastric fundus is further mobilized along the pancreaticogastric fold, where the posterior gastric artery is identified and divided.
♦ The distal stomach is mobilized by the division of the gastrocolic ligament at the greater curvature and then the attachments between the posterior gastric wall and pancreas. When the gastric duodenal artery is identified, Kocher’s maneuver is performed. The duodenum and pancreas head are detached from retroperitoneum and retracted to the patient’s left side. Care is taken to protect the gastroduodenal artery, to maintain blood flow to the right gastroepiploic artery to supply the gastric conduit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree