Imaging of the Renal Transplant Recipient
Jade J. Wong-You-Cheong
Barry D. Daly
Department of Diagnostic Radiology, University of Maryland School of Medicine, Baltimore, Maryland 21201
INTRODUCTION
This chapter shall describe, illustrate and discuss the use of imaging studies in the management of renal transplant recipients, and the role of the radiologist in the diagnosis and treatment of common and important problems that may develop in these patients. Similar to many other areas of medicine, no “one size fits all” imaging test exists for renal transplantation, and, in the last two decades there has been a rapid increase in the number and range of imaging modalities available. It is difficult for the renal physician or surgeon to be familiar with all of the options available, and a radiologist with sub specialized training or interest in this subject can be of value as a consultant. Over the same time period, the interventional radiologist has become more active in the minimally-invasive management of problems such as fluid collections, vascular thrombosis or arteriovenous fistulas, and the use of percutaneous techniques for therapy shall be discussed also. The first part of the chapter describes the different imaging modalities and their general applications, followed by a compilation of frequently encountered and important complications of renal transplantation and the specific role of different imaging studies in diagnosis and management.
IMAGING MODALITIES
Radiography
Plain films have limited utility in the post-operative management after renal transplantation, and are generally ordered for abdominal pain, distension and vomiting. Erect and supine views can distinguish paralytic ileus from obstruction. The position of nasogastric tubes and ureteral stents can also be ascertained. Allograft calculi may be visible on abdominal radiographs.
Ultrasound
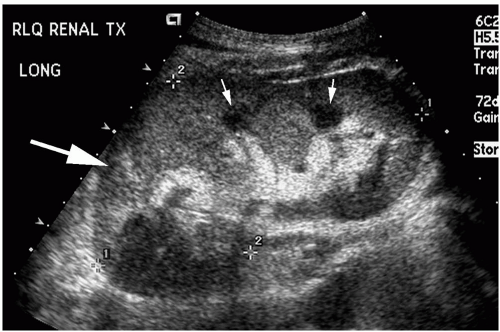
Ultrasound is the most frequently used imaging technique in renal transplantation. The superficial location of the renal transplant in the iliac fossa allows access for high resolution ultrasound imaging (Fig. 32.1). Gray scale evaluation of renal transplant morphology includes evaluation of the size and echotexture of the allograft, as well as detection of hydronephrosis, stone or mass, and post-operative complications such as fluid collections or hematomas. The bladder is also evaluated. Morphologic evaluation is supplemented by color/power and duplex Doppler evaluation for parenchymal
blood flow and patency of the renal arteries and veins. Ultrasound is an ideal initial modality for the detection and evaluation of vascular complications such as thrombosis, stenosis or arteriovenous malformations.
blood flow and patency of the renal arteries and veins. Ultrasound is an ideal initial modality for the detection and evaluation of vascular complications such as thrombosis, stenosis or arteriovenous malformations.
Normal Doppler arterial waveforms consist of continuous antegrade flow throughout the cardiac cycle with low resistance diastolic flow. Abnormal intra-renal arterial resistance can be quantified by calculation of indices such as the resistive index and the pulsatility index (Fig. 32.2). Whilst both indices reflect altered hemodynamics, the resistive index (RI) is the most commonly used. Normal resistive index ranges from 0.65-0.70 (1). RI values depend on local vascular status rather than renal function, and measurements are neither very sensitive nor specific (2). Causes of increased intra renal resistive index include rejection, acute tubular necrosis, hydronephrosis and vascular thrombosis.
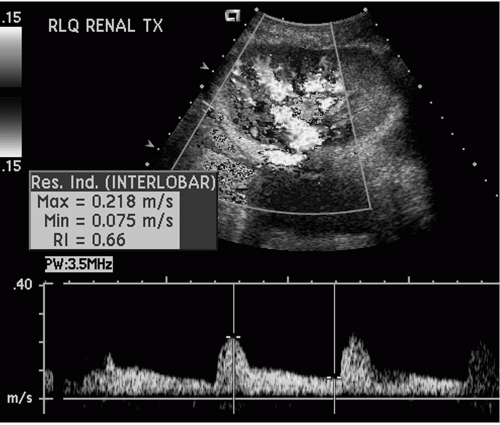 FIG. 32.2. Duplex Doppler tracing of an intrarenal artery demonstrating continuous antegrade, low resistance arterial flow throughout the cardiac cycle. The resistive index has been measured at 0.66. |
Ultrasound is a real time, multi-planar, rapidly performed modality that is widely available, and relatively cheap. It can be performed at the bedside, and as often as required and does not use ionizing radiation. Diagnostic evaluation can however, be limited by large body habitus and extensive intestinal gas. The quality of ultrasound examinations is also dependent upon the skills of the operator. The advent of ultrasound contrast media will likely increase the diagnostic value of this modality, particularly with respect to vascular evaluation (3,4). Ultrasound is highly sensitive in the detection of hydronephrosis, fluid collections and vascular complications. It is also an excellent modality for guidance of interventional procedures such as biopsy, aspiration or drainage of fluid collections, and access for antegrade pyelography or percutaneous nephrostomy placement.
Computed Tomography
Computed Tomography (CT) utilizes ionizing radiation to produce cross sectional images with high contrast resolution. In general, computed tomography is performed for symptoms and signs outside of the renal transplant, e.g. fever, leucocytosis, or gastrointestinal complaints. An optimum CT examination requires enteric contrast administered orally and intravenous iodinated contrast. However, iodinated intravascular contrast is the third most common cause of hospital acquired renal failure and its use should be judicious in renal transplant recipients (5). The incidence of contrast-induced nephropathy is higher in patients with preexisting renal impairment and diabetes. Whether renal transplantation or use of calcineurin inhibitors imparts additional risk of radiocontrast nephropathy is not clear. Elevation in serum creatinine after use of contrast agents within the early post-operative period is likely multi-factorial, with contributory causes including acute tubular necrosis, rejection, cyclosporine toxicity and dehydration (6). Pre-hydration prior to the CT study decreases the risk of contrast nephrotoxicity but does not eliminate it (5). Although never specifically or adequately evaluated in the renal transplant population, other prophylactic measures such as administration of the antioxidant acetylcysteine are employed by many clinicians in hopes of preventing or ameliorating the nephrotoxicity of contrast agents. In the post-operative renal transplant recipient, restoration of renal function may be delayed and it is therefore our practice to withhold intravenous contrast until renal function is adequate, and the use of vascular contrast considered absolutely necessary. A stable serum creatinine below 1.5 mmol/ml [1.5 mg/dL or 132 μmol/L] is our cutoff for administering intravenous contrast media in these patients. Above this level, unenhanced scans should be performed and reviewed before a decision is made to acquire additional images after intravenous contrast injection. For most indications such as fever, pain and diarrhea, an adequate
study can be obtained without intravenous contrast. If required for vascular or parenchymal detail, a contrast-enhanced CT angiogram (CTA) can be performed if renal function permits. When necessary, a reduced dose of contrast may be used. Alternatively, a magnetic resonance study may be substituted.
study can be obtained without intravenous contrast. If required for vascular or parenchymal detail, a contrast-enhanced CT angiogram (CTA) can be performed if renal function permits. When necessary, a reduced dose of contrast may be used. Alternatively, a magnetic resonance study may be substituted.
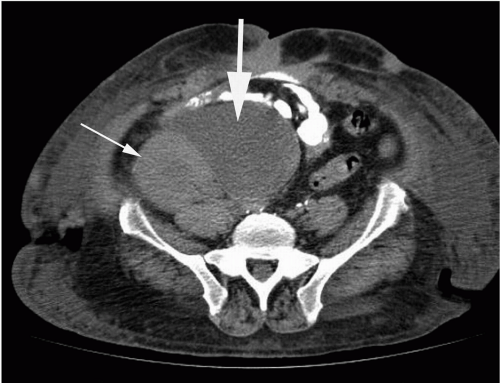 FIG. 32.3. Computed tomography performed with oral contrast only. There is a lymphocele (thick arrow) medial to the renal transplant (thin arrow). |
CT is excellent for detection and anatomic localization of fluid collections (Fig. 32.3) and for guidance of interventional procedures. Transplant biopsies may also be performed using CT guidance instead of ultrasound for deep or less accessible allografts. A non-contrast helical CT scan may be useful in evaluating for kidney stones in the native kidney or renal allograft collecting system and ureters.
Nuclear scintigraphy
Nuclear scintigraphy renal studies are of value for the assessment of renal allograft function. For many years, technetium 99m pentetate (DTPA) was the most frequently used radiopharmaceutical for evaluation of renal allografts. In the last decade, this agent has been replaced by technetium 99m mertiatade (MAG3). Technetium 99m MAG3 is an agent that undergoes predominantly tubular secretion, and is a more efficient imaging agent than technetium DTPA. Images are obtained over the kidney immediately after the intravenous injection of technetium MAG3 to assess perfusion of the transplant (7). Normal, decreased or absent allograft renal blood flow may be calculated by comparison of the intensity of radiotracer activity over the kidney with the adjacent aorta or iliac blood vessels. Glomerular filtration rate (GFR) and effective renal plasma flow (ERPF) may be calculated. However, serial studies are often necessary in the determination of abnormal renal perfusion and ideally, a baseline MAG3 study is obtained within several days of the transplantation. Imaging appearances may be compromised by poor hydration, and evaluation of the transplant may also be limited by uptake and secretion of MAG3 within the native kidneys if these remain partially functional.
Renal transplant vascular complications may be identified during the perfusion phase of technetium MAG3 studies although these abnormalities are more optimally evaluated and detected with Doppler ultrasound techniques.
Delayed scintigraphic imaging of the renal transplant is obtained after 30 minutes. The secretion of MAG3 into the renal collecting system, ureter and bladder is measured and imaged. MAG3 studies remain very helpful for the detection of urinary leak and ureteral obstruction that most often occur at the anastomosis of the transplant ureter to the recipient bladder (Fig. 32.4). MAG3 studies may also be helpful in the detection of vesico-ureteric reflux. This complication is frequently seen in transplanted kidneys due to the absence of a sphincter at the uretero-vesical junction. Radiotracer intensity over the ureter and kidney may alternate over time due to periodic reflux of radiotracer-labeled urine from the bladder into the ureter and renal pelvis.
Isotope renography can be used to investigate suspected acute tubular necrosis but is not much used for this purpose with ultrasound being the preferred modality to confirm patency of major renal vessels and absence of obstruction in the new transplant. Isotope renography is not of major value for the detection of rejection in transplant kidneys.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is a very versatile modality. MRI can be performed in multiple planes, does
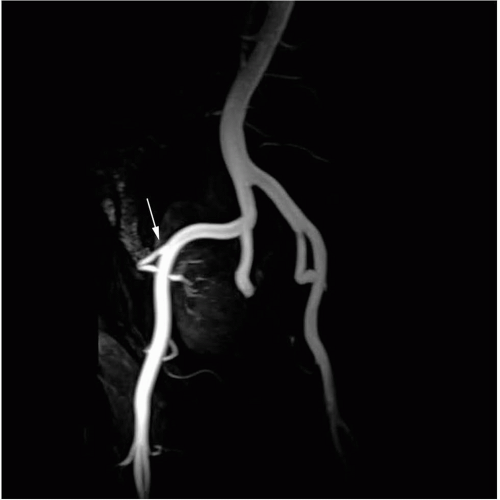
not use ionizing radiation and has high contrast resolution, making it sensitive to pathologic change within tissues. The use of varying pulse sequences enables tissue characterization (such as fluid, fat and hematoma) and differentiation of fluid collections. MR can detect flowing blood in major vessels without the use of iodinated contrast. However when performing magnetic resonance angiography (MRA), nonnephrotoxic intravenous chelates of gadolinium gadopentetate are used to increase image resolution and improve detection of small vessels. High quality angiographic images are thus acquired non-invasively. Various three-dimensional reconstruction techniques can be used to allow evaluation of complex vascular anatomy or tortuous vessels (Fig. 32.5). Intravenous MR contrast agents also improve parenchymal evaluation and aid in the characterization of renal cystic and solid masses and microabscesses.
not use ionizing radiation and has high contrast resolution, making it sensitive to pathologic change within tissues. The use of varying pulse sequences enables tissue characterization (such as fluid, fat and hematoma) and differentiation of fluid collections. MR can detect flowing blood in major vessels without the use of iodinated contrast. However when performing magnetic resonance angiography (MRA), nonnephrotoxic intravenous chelates of gadolinium gadopentetate are used to increase image resolution and improve detection of small vessels. High quality angiographic images are thus acquired non-invasively. Various three-dimensional reconstruction techniques can be used to allow evaluation of complex vascular anatomy or tortuous vessels (Fig. 32.5). Intravenous MR contrast agents also improve parenchymal evaluation and aid in the characterization of renal cystic and solid masses and microabscesses.
MRI is contraindicated in patients with pacemakers and some metallic implants such as intra-cranial aneurysm clips and heart valves. Patients with claustrophobia may be unable to tolerate MRI despite sedation. Other disadvantages of MRI include the cost, availability, and relative length of the studies. Many of the newer sequences are breath held and require patient cooperation. Artifacts from surgical clips may cause spurious stenoses (8).
Angiography
Whilst conventional angiography is invasive and requires nephrotoxic iodinated intra vascular contrast media, it is the gold standard for evaluation of arterio-venous fistulae, pseudo-aneurysms, and renal artery stenosis. In practice, vascular abnormalities are generally initially detected by Doppler ultrasound and may be confirmed by MR angiography. Conventional angiography with digital subtraction techniques is reserved for confirmation of suspected abnormalities immediately prior to subsequent percutaneous transcatheter interventions such as angioplasty and stent placement in transplant artery stenosis or embolization of a fistula (9,10).
Given the nephrotoxicity of iodinated contrast, alternatives have been used but do not produce equal radiographic image quality (11,12). Carbon dioxide gas has been used as an intra-vascular agent but digital subtraction techniques are needed to visualize the vessels due to poor image contrast (12,13). Dimeglumine-based contrast agents (developed for MRI) can also be used as angiographic contrast agents. They are excreted by the kidney via glomerular filtration. They are associated with a better renal safety profile than iodinated contrast but are more expensive and produce poorer radiographic contrast on angiographic studies (11).
Venography may be used for the detection of venous anastomotic strictures and thrombosis although, as noted above, ultrasound is a sensitive non invasive initial test which is more appropriate in the superficially placed renal transplant.
CT angiography is not routinely used in post operative renal transplant evaluation due to the potential for iodinated contrast material to cause dysfunction in the allograft, though MR angiography and venography are suitable screening techniques for detection of anatomic stricture formation or occlusion in the appropriate setting.
PRE TRANSPLANT WORK UP
At our center, patients undergoing evaluation prior to transplantation have a screening abdominal ultrasound for gallstones and a chest radiograph. Patients with analgesic nephropathy and acquired cystic disease from long term dialysis should also undergo screening of the native kidneys for renal carcinoma (14). Ultrasound is widely used initially for this purpose but if suspicious lesions are present, additional imaging work up is required for confirmation and staging. This is best done by gadolinium-enhanced MR, or enhanced CT (if the patient is on long term dialysis and able to receive intravenous contrast).
Selected patients with peripheral vascular disease are investigated with Doppler ankle brachial indices as a screening tool followed by MRA or conventional angiography to ensure availability of suitable engraftment sites. Carotid ultrasound and MRA are also selectively used.
POST TRANSPLANT IMAGING EVALUATION
Routine post-operative imaging is not performed at our institution. Common indications for imaging are: absent or decreased urine output, delayed graft function, rising or persistently elevated creatinine, fever, pain over the allograft or drop in hematocrit. Hypertension or hematuria may also prompt imaging evaluation. Patients with delayed graft function may have baseline imaging studies prior to discharge, which can be useful for comparison when follow up studies are obtained. Ultrasound is the most commonly performed modality due to its unique advantages previously described. However CT, scintigraphy and MR are complementary modalities in the radiologic armamentarium.
COMPLICATIONS AFTER RENAL TRANSPLANTATION
Immediate complications occur in the first week and are mostly related to the surgical procedure. These include renal artery or vein thrombosis, hemorrhage, ureteral edema, acute tubular necrosis and acute rejection. Early complications occur between 1 week and 1 month. These include acute rejection, urinary leak, infection, fluid collections and vascular thrombosis. After 1 month, lymphoceles, acute or chronic rejection, ureteral strictures, renal artery stenosis, infection, cyclosporine toxicity, recurrence of renal pathology, and neoplasms form the bulk of problems likely to be encountered.
The most common complications are perinephric fluid collections which occur in up to 50% of recipients (15,16).
More general complications relating to abdominal surgery include post-operative ileus, bowel obstruction, venous thromboembolic disease and infections, (either systemic, pulmonary, renal or bowel in origin). CT is the most useful imaging study when searching for infection or in patients with non specific chest or abdominal symptoms. Ultrasound is the study of choice for arm or leg deep venous thrombosis but is limited in the upper mediastinum or pelvis because the vessels may be obscured by gas. For suspected thoracic, pelvic or inferior vena cava thrombus, CT with contrast or MRI are the preferred modalities. Multislice CT has become the study of choice for pulmonary embolism in our institution but requires the use of iodinated contrast medium.
Delayed graft function
Acute tubular necrosis is the most common cause of delayed graft function in the first week after surgery. Imaging is generally performed to exclude other causes of poor graft function as there are no specific imaging characteristics of acute tubular necrosis. Allograft swelling and elevated resistive index may be observed at sonography, however ultrasound guided biopsy is diagnostic. At nuclear scintigraphy, relative preservation of perfusion with impaired clearance of tracer is noted (7,17).
Cyclosporine toxicity
Cyclosporine is nephrotoxic and can cause graft ischemia. The sonographic findings are indistinguishable from acute tubular necrosis or acute rejection. The diagnosis is made by measurement of serum cyclosporine levels but may be suggested by biopsy findings.
Fluid collections
Fluid collections detected in the early post-operative phase are usually insignificant seromas or liquefying hematomas. Significant collections occur in 11-18% of recipients, and include lymphoceles (43%), abscesses (30%), urinomas (18%) and hematomas (9%) (15). Ultrasound imaging is sensitive in the detection of collections unless they are small, deep or obscured by bowel gas. The appearance of the fluid is generally non-specific unless there is internal hemorrhage. Simple seromas or urinomas appear as anechoic collections around the transplant.
Hematomas can be of variable appearance depending on age. Acute hematomas can be hyperechoic and solid appearing and can be mistaken for pelvic fat or even bowel. With degradation of blood products, hematomas become more cystic with internal fine septations (Fig. 32.6). Intraperitoneal hemorrhage is less common but readily detectable by sonography. Retroperitoneal or deep pelvic hematomas however may be missed by ultrasound and CT is more sensitive for these and for small amounts of hemoperitoneum. Acute hemorrhage is hyperattenuating on unenhanced CT (Fig. 32.7). In urgent situations, the CT scan can be performed without delay if oral contrast is not administered.
Lymphoceles develop later, typically around 4-8 weeks, but sometimes earlier. These occur in 18% of recipients (18,19).
Symptoms include local swelling or leg swelling, graft dysfunction due to obstruction of drainage, or urinary frequency from mass effect on the bladder. Ultrasound or CT can detect lymphoceles as round or elliptical fluid collections centered on the vascular pedicle of the allograft (Fig. 32.3 and 32.8). On ultrasound, internal septations may be seen. Fluid collections may also be incidentally detected by nuclear scintigraphy as a photopenic area exerting mass effect on the kidney.
Symptoms include local swelling or leg swelling, graft dysfunction due to obstruction of drainage, or urinary frequency from mass effect on the bladder. Ultrasound or CT can detect lymphoceles as round or elliptical fluid collections centered on the vascular pedicle of the allograft (Fig. 32.3 and 32.8). On ultrasound, internal septations may be seen. Fluid collections may also be incidentally detected by nuclear scintigraphy as a photopenic area exerting mass effect on the kidney.
 FIG. 32.6. Ultrasound of a large post biopsy complex perinephric hematoma (thin arrows) medial to and compressing the renal transplant seen in transverse section (thick arrow). |
With the exception of internal gas indicating an abscess, there are no definitive imaging criteria for superimposed infection within collections. Generally, collections are aspirated under ultrasound or CT guidance for diagnosis if there is clinical suspicion for infection or urine leak. Routinely, aspirated fluid is sent for gram stain, culture and determination of urea and creatinine. Aspiration can also be therapeutic when there is mass effect on the kidney or ureter, or patient discomfort. A percutaneous drainage catheter may be placed if infection is suspected.
Lymphoceles tend to recur after simple aspiration. Currently, definitive management is surgical marsupialization, either open or laparoscopic (20). Preoperative CT is often obtained for surgical planning. Sclerotherapy with alcohol through a percutaneous catheter has fallen out of favor because of the risk of infection in the indwelling catheter during the long course of treatment, and the prolonged treatment time for the patient.
Urologic Complications
Urinary leak
This generally results from ureteric ischemia and necrosis or from technical problems at the ureteroneocystostomy (22). Occasionally, segmental parenchymal infarction can result in a caliceal fistula with urinoma (23).
Presenting features include rising creatinine, decreasing urine output, pain and swelling over the allograft or in the ipsilateral lower limb, increasing ascites, and fluid leakage from wound. A non-specific fluid collection may be found on imaging with sonography or CT and can be confirmed to represent
urine by chemical analysis of aspirated fluid. The urine leak itself can be directly visualized with a MAG3 isotope renogram demonstrating extra-urinary concentration of radioactivity in a localized urinoma or as diffuse urinary ascites. Urinary leaks may also be diagnosed by a water soluble contrast cystogram which has the unique advantage of directly demonstrating the location of the leak. Leaks are treated promptly because of risk of infection in the immunosuppressed host. A percutaneous nephrostomy or ureteral stent may be inserted pending definitive open repair and can allow accurate delineation of the site of leak (24). In a few cases, percutaneous management alone may be successful (24).
urine by chemical analysis of aspirated fluid. The urine leak itself can be directly visualized with a MAG3 isotope renogram demonstrating extra-urinary concentration of radioactivity in a localized urinoma or as diffuse urinary ascites. Urinary leaks may also be diagnosed by a water soluble contrast cystogram which has the unique advantage of directly demonstrating the location of the leak. Leaks are treated promptly because of risk of infection in the immunosuppressed host. A percutaneous nephrostomy or ureteral stent may be inserted pending definitive open repair and can allow accurate delineation of the site of leak (24). In a few cases, percutaneous management alone may be successful (24).
Hydronephrosis
Hydronephrosis is readily diagnosed by ultrasound as well as CT, nuclear scintigraphy and MRI. Early in the postoperative period, hydronephrosis may be secondary to anastomotic edema at the uretero-vesical junction, or may reflect mild non-obstructive pyelocaliectasis resulting from denervation. Extrinsic compression by fluid collections may also be a cause. An overdistended bladder may cause functional obstruction at the uretero-vesical junction. Later on, hydronephrosis can result from strictures secondary to ureteric ischemia or kinks from surgical technique, extrinsic compression by fluid collections, rejection, ureteral stenosis due to BK virus infection, or obstruction from ureteric blood clot or calculi.
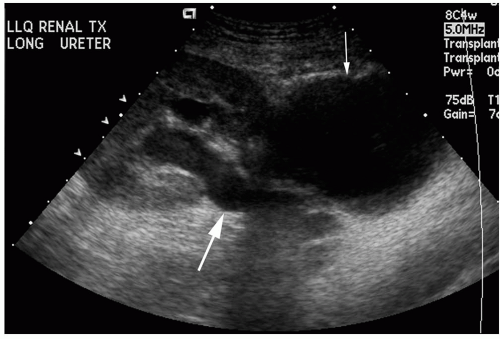
In the hydronephrotic kidney, the renal pelvis is dilated and urine filled, dividing the hyperechoic sinus fat (Fig. 32.9A). Branching dilated calyces help to distinguish this entity from a parapelvic cyst. The dilated ureter may also be visible as a tubular structure extending between the renal pelvis and the bladder. The normal transplant ureter is not usually visible.
A dilated collecting system is not necessarily obstructed. Whilst dilatation can be seen in 11.7-31% of transplants, only 4-11.8% will have an underlying obstructive lesion (25,26). Vesico-ureteral reflux, a flaccid collecting system, an extra-renal pelvis, or a persistently dilated system post obstruction or infection may be mistaken for obstruction. During periods of obstruction, renovascular impedance may increase (measurable by the resistive or pulsatility index). However this has not proven useful in distinguishing obstruction from non-obstructive dilatation (27,28). Correlation with urinary function and history may help make the distinction. Dilated calyces that persist after bladder emptying are associated with a ureteral stricture in 50% (25). Technetium 99m MAG3 studies are very helpful in distinguishing between a dilated but non-obstructed collecting system and a clinically significant obstruction due to stricture formation (Fig. 32.4). This study is performed with a
diuretic challenge (7). Less frequently a percutaneous antegrade ureterogram with pressure and flow measurements (Whittaker test) is performed for this indication. The advantage of this procedure is that drainage can be performed simultaneously.
diuretic challenge (7). Less frequently a percutaneous antegrade ureterogram with pressure and flow measurements (Whittaker test) is performed for this indication. The advantage of this procedure is that drainage can be performed simultaneously.
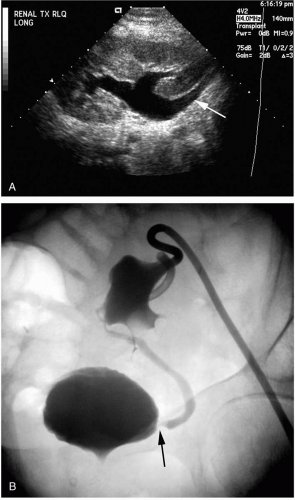 FIG. 32.9. A. Ultrasound showing hydronephrosis and hydroureter (arrow) secondary to distal ureteral stenosis. B. Percutaneous antegrade nephrostogram demonstrating a dilated collecting system and ureter secondary to a distal anastomotic stricture (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|