Up to 50% of patients with cirrhosis have esophageal varices at initial endoscopy, and nearly all patients with varices have a high portal pressure, that is, an hepatic venous pressure gradient (HVPG) of 12 mmHg or higher (normal, 3–5). In patients without varices, esophageal varices develop and grow in size at a rate of about 7% per year as a result of ongoing portal hypertension. It has been shown that varices develop at a significantly higher rate in patients with a baseline HVPG above 10 mmHg and in patients in whom the HVPG increases by more than 10% in the first year. Without treatment, varices rupture in about one third of patients, with the highest rates observed in patients with large varices, red wale marks. and/or in Child C patients. In the past, and before the use of drugs, bands, and/or shunts, 4 of 10 patients with acute variceal hemorrhage (AVH) died at 6 weeks, one third rebled at 6 weeks, and only about one third survived beyond 1 year.
This article updates the current use of drugs, bands, and shunts in the different settings—primary prophylaxis, secondary prophylaxis, and the treatment of AVH—and shows how they have altered the natural history of varices and variceal hemorrhage.
Drugs
Most currently used drugs to treat varices and/or variceal hemorrhage cause splanchnic vasoconstriction leading to a reduction in portal venous inflow and consequently to a decrease in portal pressure. Drugs in this category include nonselective β-blockers (NSBB), vasopressin and its analog terlipressin, and somatostatin and its analogs, octreotide and vapreotide. Vasodilators are another type of drugs that can reduce portal pressure through intrahepatic vasodilatation. However, most of the vasodilators currently available, specifically nitrates (which have been the most widely investigated), act not only on the intrahepatic circulation, but also have a potentially deleterious systemic vasodilating effect and seem to reduce portal pressure through reflex splanchnic vasoconstriction that results from hypotension rather than by causing intrahepatic vasodilatation. In fact, a randomized, controlled trial (RCT) of isosorbide mononitrate (ISMN) versus placebo in the prevention of first variceal hemorrhage showed a higher variceal hemorrhage rate in the ISMN group. Therefore, vasodilators used alone are not recommended in the management of portal hypertension, but are used in combination with NSBB when they have a synergistic portal pressure-reducing effect.
NSBB
Propranolol and nadolol are the most commonly used NSBB for the chronic outpatient management of varices/variceal hemorrhage. NSBB act through β-1 and β-2 adrenergic receptor blockade. β-1 Blockade leads to a decrease in portal flow by decreasing cardiac output, whereas β-2 blockade leads to a decrease in portal flow directly by inducing splanchnic vasoconstriction. The latter effect is the most important effect and explains the lack of correlation between the decrease in heart rate induced by NSBB (a β-1 effect) and the decrease in portal pressure. More recently, a role for angiogenesis in the development of portal hypertension in cirrhosis has been described and, interestingly, there is evidence that propranolol has an antiangiogenic effect.
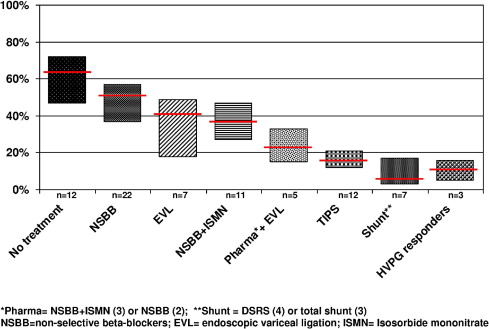
NSBBs lead to a median reduction in HVPG of approximately 15%, with 37% of the patients being hemodynamic “responders”; that is, their HVPG decreases to levels below 12 mmHg and/or is reduced by more than 20% from baseline. Hemodynamic responders have been shown to have a significantly lower incidence of variceal hemorrhage and a significantly better survival. This incidence seems to be similar to that reported for patients treated with shunt therapies [either surgical or transjugular intrahepatic portosystemic shunt (TIPS); Fig. 1 ]. Although it would seem rational to monitor the HVPG response to NSBB and adjust their dose accordingly during the therapy of varices/variceal hemorrhage, HVPG measurements are not standardized or widely used. Therefore, the currently recommended dose of NSBB is the dose that is maximally tolerated by the patient with an ideal heart rate goal of 50 to 55 bpm ( Table 1 ). It has recently been shown that NSBB dose titration performed in a nurse-led clinic results in higher maintenance doses of NSBB and a very low discontinuation rate (5%), lower than that observed in a RCT setting (∼15%).
| Therapy | Starting Dose | Therapy Goals | Maintenance/Follow-up | Comments |
|---|---|---|---|---|
| Propranolol | 20 mg orally twice a day. Adjust every 2–3 days until treatment goal is achieved. Maximal daily dose should not exceed 320 mg. | Maximum tolerated dose. Aim for resting heart rate of 50–55 bpm | At every outpatient visit, make sure that patient is appropriately β-blocked Continue indefinitely. If for primary prophylaxis, no need for follow-up EGD If for secondary prophylaxis, make sure that endoscopic procedures are scheduled. | If for secondary prophylaxis, start in hospital as soon as acute vasoconstrictor (eg, octreotide) is discontinued. |
| Nadolol | 40 mg orally once a day. Adjust every 2–3 days until treatment goal is achieved. Maximal daily dose should not exceed 160 mg. | As for propranolol. | As for propranolol. | As for propranolol. |
| Isosorbide-5-mononitrate | Only to be used in conjunction with propranolol or nadolol. 10 mg orally at night every day. Adjust every 2–3 days by adding 10 mg in the am and then in the pm. Maximal dose is 20 mg twice a day. | Maximal tolerated dose. Systolic blood pressure >95 mmHg. | Continue indefinitely. | Started after patients are on a stable maintenance dose of NSBB. Recommended only for secondary prophylaxis. |
The most common side effects related to NSBB are lightheadedness, fatigue, and shortness of breath and the prospect of experiencing these side effects detract many patients from electing to take NSBB. Additionally, up to 15% of the patients may have relative contraindications (eg, sinus bradycardia, insulin-dependent diabetes) or absolute contraindications (eg, obstructive pulmonary disease, heart failure, aortic valve disease, heart block, peripheral arterial insufficiency) to NSBB.
A recent study suggests that NSBB are associated with a poorer survival in patients with refractory ascites and that NSBB should be contraindicated in this setting. However, the study is retrospective and the groups were disparate at baseline, with patients on NSBB having more advanced disease as shown by a higher prevalence of varices and variceal hemorrhage. Contrary to this finding, in a meta-analysis of NSBB versus placebo for recurrent variceal hemorrhage, sensitivity analysis showed that NSBB were associated with a significant survival benefit in patients with the most severe liver disease. This survival benefit could be explained, at least partially, by the finding that NSBB may lower the risk of spontaneous bacterial peritonitis in patients with cirrhosis and ascites. At this time, and unless stronger evidence arises, the use of NSBB in patients with refractory ascites should not be contraindicated.
NSBB in the prevention of varices
A large, multicenter, double-blind RCT of timolol, an NSBB, versus placebo in patients with cirrhosis and portal hypertension but without varices showed a similar rate of development of varices in both treatment groups with a higher rate of adverse events in the timolol group. Therefore, NSBB are not recommended for the prevention of varices.
Notably, in this very compensated group of patients, treatment of the underlying cause of cirrhosis (alcohol abstention, antiviral therapy) is the mainstay of therapy, because treating the etiology can potentially reduce portal pressure, thereby reducing variceal development.
NSBB in the prevention of first variceal hemorrhage
Overall, NSBB compared with placebo or no active drug decreases the risk of the first episode of variceal hemorrhage (over 2 years) by about 50%. In patients with medium/large varices, who are at the greatest risk of first variceal hemorrhage, propranolol or nadolol significantly reduce the risk of first variceal hemorrhage, from 30% to 14% at a median follow-up of 2 years. Only 11 patients need to be treated to prevent 1 bleeding episode.
Meta-analyses of trials comparing NSBB versus endoscopic variceal ligation (EVL) show that EVL is more effective in preventing first variceal hemorrhage, without differences in mortality. However, meta-analyses restricted to trials with an adequate design or with a sample size greater than 100 show no differences in the rate of first variceal hemorrhage between NSBB and EVL. Therefore, because both therapies seem equal, it has been recommended that the choice should depend on local resources, patient preferences, and presence of contraindications to either therapy.
NSBB have advantages that go beyond the prevention of first variceal hemorrhage. In this setting, reductions in HVPG of only less than 10% to 12% from baseline have been associated with a decrease in the development of ascites, spontaneous bacterial peritonitis, and death. This is not surprising; as these complications result at least partially from portal hypertension. Therefore, it is reasonable to start with NSBB and switch to EVL in cases of intolerance to NSBB.
Patients with small varices with red wale marks or that are present in a Child C patient have the same (or an even greater) risk of hemorrhage than patients with large varices and are therefore considered “high risk.” Because EVL is not feasible in many of these cases, NSBB are the currently recommended therapy. In other patients with small varices, nadolol was shown to significantly decrease progression to large varices and in these patients NSBB could be administered, although this is considered optional. Once patients are started on NSBB, there is no need to perform follow-up endoscopies.
NSBB in the prevention of recurrent variceal hemorrhage
If patients who have recovered from an episode of AVH receive no therapy, the risk of recurrent variceal hemorrhage is very high at around 65% in 1 to 2 years. NSBB significantly reduce the risk of rebleeding, and prolong survival. Only 5 patients need to be treated with a NSBB to prevent 1 rebleeding episode.
The efficacy of NSBB has been shown to correlate with hemodynamic response. In this setting, a reduction in HVPG of more than 20% from the baseline value, or to values of 12 mmHg or lower, is associated with a very low rebleeding rate of approximately 11%, a rate comparable to that associated with shunting therapies ( Fig. 1 ). HVPG-guided therapy cannot yet be recommended in daily practice, not only owing to the lack of standardization of HVPG measurements, but also because of uncertain issues such as the best timing (or need) for repeat HVPG measurement and the best treatment for hemodynamic nonresponders.
The combination of pharmacologic therapy (NSBB with or without ISMN) plus EVL seems to be more effective than either therapy alone ( Fig. 1 ). NSBB plus EVL remains the recommended first-line treatment to prevent recurrent variceal hemorrhage.
As a rule, NSBB should be initiated once variceal hemorrhage is controlled and acute intravenous vasoactive drugs have been discontinued; that is, between 2 and 5 days after the initiation of intravenous therapy.
NSBB Plus Nitrates
In hemodynamic studies, the association of vasodilators such as ISMN or prazosin with NSBB leads to a greater reduction in HVPG (20%–24%) compared with the reduction observed with NSBB alone. However, complications, mainly ascites and/or symptomatic hypotension, occur more frequently with combination therapy. The rate of HVPG responders with NSBB plus ISMN is 44%, a rate that is significantly higher than that observed with NSBB alone (37%). In RCT, a meta-analysis has confirmed a significantly higher number of side effects for NSBB plus ISMN (38%) compared with NSBB alone (23%), with a higher discontinuation rate (15% vs 6%, respectively). Recommended doses of ISMN are shown in Table 1 .
NSBB plus ISMN in the prevention of first variceal hemorrhage
The largest, double-blind RCT comparing propranolol plus placebo versus propranolol plus ISMN showed no differences in the rate of first variceal hemorrhage or mortality between groups. These findings have been confirmed in a recent Cochrane meta-analysis. Therefore, the combination of NSBB plus nitrates is not recommended in the primary prophylaxis of variceal hemorrhage.
NSBB plus nitrates in the prevention of recurrent variceal hemorrhage
A single RCT fully published in English compared propranolol alone versus propranolol plus ISMN and showed, at the end of the study, a borderline significant difference in favor of combination therapy regarding prevention of recurrent variceal hemorrhage ( P = .05). This difference became significant after an additional year of follow-up ( P = .03), without differences in the incidence of overall rebleeding. A recent Cochrane meta-analysis confirms no differences in the rate of overall bleeding or mortality between these therapies, but a borderline lower rate of recurrent variceal hemorrhage in patients on combination NSBB plus nitrates (relative risk, 0.71; 95% confidence interval, 0.52–0.96) and with a higher rate of side effects.
Compared with endoscopic therapy (sclerotherapy or EVL), NSBB plus ISMN show no differences regarding recurrent hemorrhage, but seem to reduce mortality, although this effect was not confirmed in trial sequential analysis. This effect on mortality is consistent with results from one of the studies included in the meta-analysis that shows that, in the long term (82-month follow-up) propranolol plus ISMN is associated with a better survival compared with EVL.
The combination of NSBB with ISMN has similar rates of recurrent hemorrhage compared with EVL alone, whereas the combination of pharmacologic (NSBB alone or NSBB with ISMN) plus EVL has lower rebleeding rates than either therapy alone, consistent with results of a recent meta-analysis and a recent summary of trials. Therefore, the recommended therapy for prevention of recurrent variceal hemorrhage is EVL plus pharmacologic therapy. Because the combination of NSBB and ISMN has more adverse events than NSBB alone and, until a survival advantage of NSBB and ISMN can be confirmed, the recommended pharmacologic therapy to be associated to EVL is NSBB alone.
Parenteral Vasoconstrictors in the Treatment of Acute Variceal Hemorrhage
In the setting of AVH, NSBB are not indicated and have not been tested given their slow action and a potentially deleterious effect blocking the heart rate response to hypovolemia. Intravenously administered splanchnic vasoconstrictors that lower portal pressure acutely are vasopressin (and its analog terlipressin) and somatostatin (and its analogs octreotide and vapreotide). Parenteral vasoconstrictors, particularly if safe, are generally applicable and can be initiated as soon as a diagnosis of variceal hemorrhage is suspected, even before diagnostic esophagogastroduodenoscopy (EGD).
Vasopressin is the most potent splanchnic vasoconstrictor. It reduces blood flow to all splanchnic organs, leading to a decrease in portal venous inflow and a decrease in portal pressure. Unfortunately, the clinical usefulness of vasopressin is limited by its multiple side effects that are related to splanchnic vasoconstriction (eg, bowel ischemia) and to systemic vasoconstriction (eg, hypertension, myocardial ischemia). Combining vasopressin with transdermal glyceryl trinitrate has been shown to reduce some of these side effects. However, because other much safer drugs are now available, vasopressin plus nitroglycerin is only recommended when these are unavailable.
Terlipressin is a synthetic analog of vasopressin that has a longer biological activity and significantly fewer side effects than vasopressin. It is the only vasoconstrictor that has shown a survival benefit when compared with placebo.
Somatostatin and analogs such as octreotide and vapreotide also cause splanchnic vasoconstriction at pharmacologic doses, both through an inhibition of the release of vasodilatory peptides (mainly glucagon) and a local vasoconstrictive effect. Bolus injections of both somatostatin and octreotide cause a marked reduction in portal pressure but, with continuous infusion, only somatostatin seems to maintain a portal hypotensive effect. Somatostatin and its analogs may have an added benefit of decreasing the postprandial (including blood in stomach) elevation in portal pressure. The near absence of side effects of somatostatin and analogs represents a major advantage over other vasoconstrictive agents, allowing them to be administered for a longer period of time.
RCTs comparing different pharmacologic agents demonstrate no differences among them regarding control of hemorrhage and early rebleeding, although vasopressin is associated with more adverse events. In practice, the choice of pharmacologic agent is usually based on availability and cost. Octreotide, a somatostatin analog, is the only safe vasoactive drug available in the United States. Doses and schedules for the different vasoconstrictors are shown in Table 2 .
| Therapy | Initial Dose | Therapy Goals | Maintenance Dose/Follow-up | Comments |
|---|---|---|---|---|
| Octreotide | IV bolus of 50 μg. Start upon suspicion of variceal hemorrhage (before endoscopic confirmation). | Control of AVH | IV infusion of 50 μg/h Duration: 2–5 days A repeat bolus of 50 μg can be administered if rebleeding or failure | Only available IV vasoconstrictor in the US (used off label for this indication) |
| Somatostatin | IV slow bolus of 250 μg. Start upon suspicion of variceal hemorrhage (before endoscopic confirmation). | Control of AVH | IV infusion of 250 μg/h Increase dose to 500 μg/h if rebleeding or failure | Not available in the US |
| Terlipressin | For the first 48 hours: boluses of 2 mg IV every 4 hours. Start upon suspicion of variceal hemorrhage (before endoscopic confirmation). | Control of AVH | After first 48 hours: 1 mg IV every 4 hours Total duration: 2–5 days | Not available in the US |
| Vapreotide | Bolus of 50 μg IV bolus. Start upon suspicion of variceal hemorrhage (before endoscopic confirmation). | Control of AVH | IV infusion of 50 μg/h Duration: 2–5 days A repeat bolus of 50 μg can be administered if rebleeding or failure | Not available in the US |
| Vasopressin plus nitroglycerin | IV infusion at 0.2–0.4 U/min (maximal dose is 0.8 U/min). Always given with IV infusion of nitroglycerin 40 μg/min (maximal dose is 400 μg/min). | Control of AVH | Adjust nitroglycerin systolic blood pressure >90 mmHg Maximal duration 24 hours | Very rarely used because of high rate of side effects Given toxicity should only be initiated once variceal source of hemorrhage is confirmed |
A meta-analysis of RCTs comparing vasoactive drugs versus sclerotherapy in the control of AVH did not find any differences in control of bleeding, 5-day failure rate, rebleeding or mortality, with a significantly higher rate of adverse events with sclerotherapy. However, another meta-analysis shows that the use of parenteral vasoconstrictors (mostly octreotide) plus endoscopic therapy is more effective than endoscopic therapy alone ; therefore, the combination of pharmacologic and endoscopic therapy is the most rational approach in the treatment of AVH.
New Drugs
Currently, fewer than half of the patients treated with NSBB and nitrates are hemodynamic responders. If this proportion could be increased with new drugs, outcomes could be improved and the need to measure HVPG to monitor response to therapy would no longer be an issue.
New drugs that have been tested in the treatment of portal hypertension are mainly those that, at least theoretically, decrease intrahepatic vascular resistance either by blocking adrenergic activity or by increasing the delivery of nitric oxide to the intrahepatic circulation. Unfortunately, vasodilators may also produce systemic vasodilatation with aggravation of the hyperdynamic circulatory state, leading to sodium retention and renal vasoconstriction.
Carvedilol is a NSBB with added vasodilatory effect through anti-α 1 adrenergic activity. At a mean dose of 31 mg/d it has a significant portal pressure-reducing effect (19%, with 58% being HVPG responders), but can also decrease arterial pressure and lead to fluid retention. However, a recent RCT on primary prophylaxis of variceal hemorrhage that compared carvedilol at a lower dose (maximum 12.5 mg/d) with EVL showed a significantly lower rate of first variceal hemorrhage with carvedilol (10%) compared with EVL (23%), without differences in mortality or side effects. However, the study has some problems and the hemorrhage rates are within the described rates of first variceal hemorrhage in RCTs of NSBB (7%–46%) or EVL (0%–25%). Therefore, carvedilol is considered a promising alternative that needs to be further explored before it can be widely recommended.
Another drug that is promising based on proof-of-concept studies but that has not been tested in RCT looking at clinical end points is simvastatin, which acts on portal pressure by improving liver generation of the vasodilator nitric oxide and by improving hepatic endothelial dysfunction in patients with cirrhosis. In a hemodynamic, placebo-controlled study, simvastatin at a dose of 20 to 40 mg/d was shown to reduce HVPG modestly (8% compared with no change in patients randomized to placebo). This effect occurred without changes in portal flow, indicating that the effect was due to a decrease in intrahepatic resistance. Results of ongoing RCTs assessing its effect combined with NSBB on clinical end points are eagerly awaited, but until then its use cannot be widely recommended.
Although angiotensin receptor blockers and angiotensin-converting enzyme inhibitors have been shown to reduce portal pressure, their use is associated with decreased creatinine clearance and they are therefore not recommended. However, a recent meta-analysis of individual patient data of studies that used these drugs shows that, in patients with Child A cirrhosis, angiotensin receptor blockers and angiotensin-converting enzyme inhibitors reduce portal pressure with minimal side effects, with deleterious effects being mostly observed in patients with decompensated cirrhosis.
Other vasodilators that have been tested recently are sildenafil, a phosphodiesterase inhibitor, and NCX-1000 [2(acetyloxy) benzoic acid-3(nitrooxymethyl)phenyl ester], a nitric oxide-releasing derivative of ursodeoxycholic acid. Both had shown selective vasodilatory effect on intrahepatic circulation in animal models of cirrhosis. However, in hemodynamic patient studies, neither reduced HVPG significantly, although both were associated with a significant, potentially deleterious, decrease in blood pressure.
Prophylactic antibiotics are non-vasoactive drugs that have been shown to reduce the rate of bacterial infections and to improve survival in patients with AVH. This effect is partially due to a decrease in the early rebleeding rate. Their use in the setting of AVH is considered standard of care.
Bands
Esophagogastroduodenoscopy (EGD) is an essential procedure for the diagnosis, grading, and treatment of varices and variceal hemorrhage. The endoscopic method of choice in the therapy of varices and variceal hemorrhage is EVL, which consists of the placement of rubber bands around varices so that they will undergo necrosis and will eventually disappear.
In expert hands, EVL is highly effective for controlling bleeding and for the eradication of varices. However, because it is a local therapy that has no effect on portal pressure, varices always recur after endoscopic eradication. It is, therefore, necessary to carry out lifelong surveillance and repeat EVL when varices recur.
EVL has essentially replaced the use of endoscopic sclerotherapy based on 2 meta-analysis of RCTs comparing EVL versus sclerotherapy in secondary prophylaxis of variceal hemorrhage, both of which showed a superiority of EVL in preventing recurrent variceal hemorrhage, with fewer complications and a lower number of endoscopic sessions needed to achieve eradication. Notably, the combination of EVL and sclerotherapy has been shown to confer no advantages over EVL alone and has a higher complication rate. Therefore, evidence accumulated so far should discourage the use of combination EVL plus sclerotherapy.
The technique of EVL uses multiband devices with application of the bands started at the gastroesophageal junction progressing upward in a spiral manner for approximately 5 to 8 cm. An average of 3 banding sessions (each performed every 1–2 weeks) are required to achieve eradication of varices (disappearance of varices or varices that are too small to be sucked into the banding device). After varices are eradicated, the first surveillance endoscopy should be performed 3 months later and, if negative, surveillance can be lengthened to every 6 months ( Table 2 ).
Bands in the Prevention of First Variceal Hemorrhage
The finding that EVL was superior to sclerotherapy in the secondary prophylaxis of variceal hemorrhage prompted the performance of prospective studies to assess its efficacy in preventing first variceal hemorrhage (primary prophylaxis). Initial studies comparing EVL with no treatment were unjustified because they ignored the fact that an effective therapy (NSBB) already existed at the time.
Even though meta-analyses of RCTs comparing EVL versus NSBB show a benefit of EVL in preventing first variceal hemorrhage, when trials with an appropriate treatment allocation, a longer follow-up, or that include a reasonable number of patients (>100) are analyzed, this borderline benefit of EVL disappears. Because both NSBB and EVL seem to be equally effective in preventing first variceal hemorrhage, the side effects of each become an important issue. Although the number of side effects is greater with NSBB than with EVL, it is the quality of side effects that is more important. Whereas no lethal side effects have been reported with the use of NSBB, 3 deaths resulting from EVL-induced bleeding ulcers were reported in these trials. Nevertheless, no differences in mortality between treatment groups have been shown in any of these meta-analyses.
This has led to the recommendation that either NSBB or EVL are reasonable options in the primary prophylaxis of variceal hemorrhage. The choice should be based on local resources and expertise, patient preference and characteristics, side effects, and contraindications. Despite this recommendation, there are currently centers that perform predominantly EVL; others prefer the more rational approach of starting with NSBB and switching to EVL if there is intolerance to NSBB.
A very recent trial compared EVL plus nadolol versus nadolol alone and reported that the combination treatment did not enhance effectiveness of primary prophylaxis, while increasing adverse events. Therefore, in this setting combination therapy is not currently recommended.
Bands in the Treatment of AVH
Two meta-analyses have shown that EVL and sclerotherapy are equally effective in controlling AVH with control rates of 80% to 90% ( Table 3 ). However, in a more recent RCT comparing somatostatin plus either sclerotherapy or EVL, the incidence of patients surviving without therapeutic failure was significantly greater in patients randomized to EVL. Serious side effects were also less common with EVL. Therefore, EVL is the recommended form of endoscopic therapy for acute esophageal variceal bleeding, although sclerotherapy may be used in the presence of active hemorrhage if ligation is technically difficult.






