Therapeutic outcome
Definition
Response to corticosteroids
Clinical improvement after treatment with high-dose oral steroids (40–60 mg prednisone or equivalent) within 30 days or clinical improvement after treatment with high-dose intravenous steroids within 7–10 days
Corticosteroid refractory (steroid refractory)
Patients who fail to respond in the time frame described above
Corticosteroid dependent (steroid dependent)
Patients who initially respond to corticosteroids but then relapse during tapering or shortly after drug discontinuation of corticosteroids and require reintroduction of corticosteroid therapy to maintain symptom control
The risks of long-term corticosteroid therapy including osteoporosis, pathological fractures, cataract, metabolic changes, acne, striae, hirsutism, psychological disturbance, and infection outweigh their benefits in some patients. Management of steroid-dependent and steroid-refractory UC may include stepped-up medical treatment or surgery. At any time, approximately 20–34 % of patients with UC have chronic active disease requiring several courses of corticosteroids to achieve remission [1, 3]. This chapter will discuss the management of steroid-dependent UC followed by the management of steroid-refractory UC.
Management of Steroid-Dependent UC
Immunosuppression with thiopurines is a mainstay in managing steroid-dependent UC. Thiopurine therapy, although more widely used in steroid-dependent Crohn’s disease (CD), may be useful for patients with steroid-dependent as well as steroid-refractory UC. Ardizzone et al. randomized 72 patients with steroid-dependent UC to receive azathioprine (AZA) 2 mg/kg daily or oral 5-aminosalicylic acid (5-ASA) 3.2 g daily for a 6-month follow-up period [4]. Steroid dependence was defined as a requirement for steroid therapy ≥10 mg daily during the preceding 6 months, with at least two attempts to discontinue the medication. They found that significantly more patients in the AZA than in the 5-ASA group had clinical and endoscopic remission and discontinued steroid therapy (53 vs. 21 %, P = 0.003).
More recently, a cohort study of 42 patients with steroid-dependent UC initiated on AZA therapy with a steroid taper showed the proportion of patients remaining in steroid-free remission at 12, 24, and 36 months was 55, 52, and 45 %, respectively [5]. AZA was dosed at 50 mg daily for the first 15 days followed by a target dose of 2–3 mg/kg daily. Steroid-dependent UC was defined as the inability to successfully reduce steroids below the equivalent of prednisone 10 mg daily within 3 months of starting steroids, relapse within 3 months of stopping steroids, or symptoms only controlled by continued use of steroids requiring a daily oral dose of 15–25 mg of prednisone for at least 6 months.
In light of these and several other studies, the American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA) recommend that patients with corticosteroid-dependent inflammatory bowel disease (IBD) be treated with AZA 2–3 mg/kg daily or 6-mercaptopurine (6-MP) 1.0–1.5 mg/kg daily in an attempt to lower or eliminate corticosteroid use (grade A) [6, 7].
The metabolites of AZA and 6-MP may influence the management of patients with IBD. AZA is nonenzymatically converted to 6-MP, which may be activated through several enzymatic steps to the active metabolites, 6-thioguanine nucleotides (6-TGN). 6-MP may also be metabolized to 6-methylmercaptopurine (6-MMP) by the enzyme thiopurine methyltransferase (TPMT) and to 6-thiouric acid (6-TU) by the enzyme xanthine oxidase. Both 6-MMP and 6-TU are inactive metabolites. (Please see Fig. 28.1.) TPMT activity may be both genetically determined and inducible, and a deficiency of this enzyme may lead to myelosuppression.
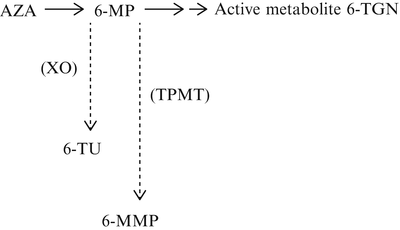

Fig. 28.1
Metabolism of azathioprine
The toxicity induced by AZA and 6-MP in patients with IBD includes bone marrow suppression in 10 % of patients (secondary to elevated 6-TGN levels) [5], pancreatitis in 3.3 % of patients, allergic reactions in 2 % of patients, drug hepatitis in 0.3 % of patients (secondary to elevated 6-MMP levels), infection in 7.4 % of patients, and neoplasm in 3.1 % of patients [8]. Routine monitoring of complete blood count with differential weekly for 4 weeks, biweekly for 4 weeks, and then every 1–2 months for the duration of treatment is suggested [9]. The ACG and AGA recommend measuring liver function tests periodically and assessing TPMT genotype or phenotype before initiation of therapy with AZA or 6-MP to detect individuals with low enzyme activity who may be at risk for myelosuppression [6, 7]. Roblin et al. performed a cross-sectional worldwide survey and showed that the use of TMPT phenotype and genotype testing was performed in only 43 and 30 % of responding gastroenterologists, respectively [10].
Dubinsky et al. showed that clinical response was highly correlated with 6-TGN levels (P < 0.0001) in pediatric patients with IBD. Further, they showed that the frequency of therapeutic response increased at 6-TGN levels >235 pmol/8 × 10 [8] erythrocytes (P < 0.001) [11]. Other studies have found that 6-MP dose is weakly associated with 6-TGN levels [12, 13]. As a result of the discrepancy in data, a controlled study to evaluate the utility of these tests is needed before recommendations for clinical use can be instituted. Thiopurine testing may play a role in assessing nonadherence or to explain therapeutic failure in patients taking AZA or 6-MP.
The antimetabolite methotrexate (MTX) is widely used in certain autoimmune diseases such as rheumatoid arthritis and psoriasis. A pilot study by Kozarek et al. in 1989 showed that 25 mg/week intramuscular injection of MTX for 12 weeks resulted in a significant reduction in prednisone dosage in patients with refractory UC or CD [14]. The chronic UC activity index decreased from 13.3 to 6.3 (P = 0.007); however, no patient with UC showed complete histological or endoscopic remission. This early study also addressed the side effects of MTX, including nausea and diarrhea, liver function test increase, leukopenia, brittle nails, and hypersensitivity pneumonitis.
Baron et al. in 1993 evaluated the efficacy and safety of oral MTX 15 mg daily in patients with refractory IBD for 18 weeks [15]. Refractory IBD was defined as a chronic active disease that lasts for more than 6 months and the failure to respond to steroids or the requirement of 20 mg/day of prednisone or more with disease flare when the dose was tapered. Ten of the 11 UC patients included were previously unsuccessfully treated with ASA or 6-MP. UC patients treated with low-dose MTX showed a statistically significant decrease in daily prednisone dose from 26.3 to 12.7 mg (P < 0.001) and a decrease in sigmoidoscopic score (graded on a scale of 0–15 based on mucopus, friability, vascular pattern, granularity, and erythema) from 10.9 to 8.0 (P < 0.003). Mild side effects including mouth ulcers, alopecia, insomnia, facial flushing, nausea and vomiting, and transient decrease in night vision were reported. This pilot study supports the previous study by Kozarek et al. concluding that low-dose oral MTX is reasonably safe as a steroid-sparing agent in patients with refractory IBD.
Oren et al. randomized 67 patients with active UC to receive oral MTX 12.5 mg weekly or placebo for 9 months [16]. Disease chronicity was defined as the requirement of steroid therapy for at least 4 of the preceding 12 months. At each visit the Mayo Clinic score was calculated, and a sigmoidoscopy was performed every 3 months. There was no significant difference in achieving remission and complete steroid withdrawal between patients assigned to MTX and patients assigned to placebo (47 vs. 49 %, P = 0.87).
In a non-placebo-controlled study, Paoluzi et al. treated steroid-dependent UC patients with oral AZA 2 mg/kg followed by intramuscular MTX 12.5 mg/week if intolerant or not responding to AZA [17]. Disease activity was monitored monthly and colonoscopy with histology performed at 3 months, 6 months, and then every 6 months thereafter. In the short-term treatment, achieving complete remission and demonstrating improvement on AZA was similar to MTX (69 vs. 60 %, 20 vs. 40 %, respectively). Of the patients who achieved complete remission in the short-term treatment, 12 of 22 patients taking AZA and all 6 patients taking MTX remained in remission at the end of long-term treatment (54 % vs. 100 %, P < 0.05). This study confirms the beneficial role of AZA in refractory UC and shows that MTX may be effective in those unresponsive or intolerant to AZA. A limitation to this study is the small study population.
It is important to mention that the dose of MTX used to treat patients with CD successfully (25 mg IM weekly) has not been tried in patients with UC. The use of lower-dose MTX has shown some promise in the studies discussed and may be of potential benefit in patients with UC. Although evidence supports the use of MTX for induction and remission with corticosteroid withdrawal in active CD, the ACG and AGA state that there is insufficient evidence to support the use of MTX in patients with active UC [6, 7].
Mycophenolate mofetil (MMF) is another drug that shows potential in steroid-dependent UC. Similar to MTX, MMF reduces the production of interferon gamma by T cells. MMF is traditionally used to prevent graft rejection in organ transplant patients. An early trial randomized patients with CD to receive AZA (2.5 mg/kg) plus 50 mg prednisolone orally versus MMF (15 mg/kg) plus 50 mg prednisolone orally [18]. Corticosteroid dosage was titrated weekly to a maintenance dose of prednisolone 5 mg daily. This study showed that treatment of patients with a Crohn’s disease activity index (CDAI) greater than 300 with MMF plus corticosteroids had a greater decrease in CDAI score during the first month of treatment compared to patients in the AZA plus corticosteroid treatment arm. Over 6 months this resulted in a greater number of MMF-treated patients entering remission. Additionally, this study showed that there were no severe adverse events in either group. Two patients in the MMF-treated group developed drug exanthema and vomiting.
Shortly after, Orth et al. carried out a prospective study to compare MMF versus AZA in patients with chronic active UC [19]. In a similar fashion, they randomized 24 patients with UC to MMF (20 mg/kg) plus prednisolone orally or AZA (2 mg/kg) plus prednisolone orally. They found that the number of patients not requiring steroids was higher in the AZA plus prednisolone group than in the MMF plus prednisolone group. Further, there were no severe adverse events reported in the AZA plus prednisolone group but two severe adverse events observed in the MMF plus prednisolone group (recurrent upper airway infections in one patient and bacterial meningitis in another patient). The authors conclude that AZA plus prednisolone appears to be more effective and more safe compared to MMF plus prednisolone in patients with UC. This trial employed dosages lower than those typically used in renal transplantation and may explain why diarrhea, hematological toxicity, primary neutropenia, or thrombocytopenia was not seen. A later study showed adverse events that include malaise, irritability, depression, joint pain, skin rash, pancreatitis, alopecia, diarrhea, and abnormal liver function tests [20]. As a result of conflicting data, the ACG and the AGA do not recommend the use of MMF in patients with steroid-dependent UC [6, 7]. If a patient has a contraindication to AZA, then MMF may be a reasonable alternative; however, further studies are essential to evaluate the effects of MMF in active UC.
The immunosuppressant tacrolimus is a calcineurin inhibitor currently approved for the prophylaxis of organ rejection in patients receiving allogeneic liver or kidney transplants. Tacrolimus may also inhibit interleukin 2 (IL-2) and therefore play a role in the pathogenesis of IBD. A retrospective study in 2006 observed 53 patients with steroid-refractory or steroid-dependent IBD [21]. The study included 40 patients with UC, 11 patients with CD, and 2 patients with pouchitis. Patients had previously failed or not tolerated therapy with 5-ASA, AZA, MTX, infliximab, cyclosporine, MMF, and budesonide in the UC group. In those patients with UC, 57 % had pancolitis, 30 % had left-sided colitis, 10 % had other parts of their colon affected, and 2.5 % had proctitis. Tacrolimus was administered orally at an initial dose of 0.1 mg/kg per day in two divided doses with goal serum trough levels of 4–8 ng/mL. At 39 months, 27 of 40 patients (67.5 %) in the UC group were in remission, 2 of 40 patients (5 %) did not respond, and 2 of 40 patients (5 %) withdrew. A reduction or discontinuation of prednisolone was achieved in 33 of 36 UC patients (91.7 %) receiving prednisolone. It is important to note that 77 % of all IBD patients included in the study were receiving concomitant AZA. Nine of 40 UC patients (22.5 %) underwent colectomy for intractable bleeding, premalignant or malignant polyps, intractable paresthesias, or the desire of the patient to have surgery rather than continue immunosuppression. Colectomy-free survival in UC patients was 56.5 % at 43 months. There were no reported side effects in 75 % of all IBD patients included. They did have opportunistic infections in 3 % of patients, increase in creatinine in 7.5 % of patients, hyperkalemia in 1.9 % of patients, hypertension in 1.9 % of patients, and tremor or paresthesias in 9.4 % of patients. The authors conclude that tacrolimus appears safe and effective in refractory IBD.
In a prospective randomized controlled trial, patients with active refractory UC were assigned to tacrolimus with a high trough concentration (10–15 ng/ml), low trough concentration (5–10 ng/ml), or placebo [22]. Patients were permitted to continue 5-ASA drugs or steroids during the study as long as the dosage was not adjusted; however, AZA or 6-MP concomitant use was prohibited. An improvement in the disease activity index (DAI) score was observed in 68.4 % of patients in the high trough concentration group compared to 10 % in the placebo group at week 2 (P < 0.001). Clinical remission was observed in 4 of 20 patients (20 %) of the high trough concentration group, 2 of 19 patients (10.5 %) of the low trough concentration group, and 1 of 17 patients (5.9 %) in the placebo group at week 2. Mucosal healing was achieved in 15 of 19 patients (78.9 %) in the high trough concentration group, 8 of 18 patients (44.4 %) in the low trough concentration group, and 2 of 16 patients (12.5 %) in the placebo group at week 2. In an open-label extension, 55.2 % of patients receiving tacrolimus therapy showed an improvement in the DAI score at week 10. The mean dose of prednisolone was reduced at week 10. Reported adverse events in this trial included: finger tremor, headache, serious viral gastroenteritis, decreases in serum magnesium, and increases in serum creatinine. This study concluded that oral tacrolimus, with optimal target trough concentration of 10–15 ng/ml, appears to be efficacious and safe as therapy in refractory UC. As a result of few data, the ACG and the AGA do not recommend tacrolimus at this time for the management of steroid-dependent or steroid-refractory UC (Table 28.2) [6, 7].
Table 28.2
Medical therapy for steroid-dependent UC
Medical therapy | Adverse events | References |
|---|---|---|
Thiopurines | Bone marrow suppression (elevated 6-TGN levels) | Chebli et al. [5] |
AZA | Infection | Kornbluth et al. [6] |
6-MP | Pancreatitis | Lichtenstein et al. [7] |
Neoplasm | Present et al. [8] | |
Allergic reactions | ||
Drug hepatitis (elevated 6-MMP levels) | ||
MTXa | Neutrophilic dermatitis (Sweet’s syndrome) | Baron et al. [15] |
Dermatitis | Oren et al. [16] | |
Leukopenia | Paoluzi et al. [17] | |
Headache | ||
Mouth ulcers | ||
Ulceration of nasal mucosa | ||
Alopecia | ||
Insomnia | ||
Facial flushing | ||
Nausea, vomiting | ||
Transient decreased night vision | ||
MMFa | Diarrhea | Neurath et al. [18] |
Neutropenia | Orth et al. [19] | |
Thrombocytopenia | Palaniappan et al. [20]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



