Fig. 37.1
Rectovaginal fistula sites. a Low, b mid, chigh. (Modified from: http://en.wikipedia.org/wiki/File:Rectovestibular_fistula_in_females.jpg by adding arrows, letters, and a legend. Under creative commons attribution 2.0 generic license, we are free to modify it:This is an open access article distributed under the terms of the creative commons attribution license (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited)
Examination under anesthesia is critical to locate the fistula, assess the quality of surrounding tissue and the presence of associated pathology, with high fistulas being the most difficult to diagnose. A palpable depression in the anterior midline of the rectum, or a visible pit like defect could be the only appreciable sign if the fistula is small. These changes may be palpable or visible on anoscopy. On vaginal examination, the darker mucosa in the fistula track may be apparent, contrasting with the light vaginal mucosa [2]. There may be visible stool or signs of vaginitis. Probing the tract is very painful and should only be done under anesthesia to avoid creating false tracts. An assessment of anal sphincter integrity and function will assist in surgical planning. This may be attainable with a good history and physical examination; however, some women may have difficulty in distinguishing incontinence from fistulous drainage. Incontinence may be caused by the fistula, an underlying disease state, or anal sphincter defect. Determining the cause of incontinence is important prior to operative intervention for a RVF [3, 4]. Supplemental studies may be necessary to confirm the presence of a fistula or to determine the extent of underlying disease.
Endorectal and transvaginal ultrasounds may be used to identify a low fistula tract [5, 6]. Alternatively, a vaginal tampon can be inserted followed by instillation of a methylene blue enema. The tampon is removed after retaining the enema for 15–20 min. If there is no staining, the diagnosis of RVFs is highly unlikely. More proximal fistulas are best diagnosed with vaginography, a barium enema or computed tomography(CT)-scan with rectal contrast. An endoscopy is necessary if inflammatory bowel disease is suspected. Biopsies under anesthesia are useful in patients with history of prior radiation to rule out malignancy. Manometry may be used to determine functional sphincter defects in the absence of an anatomic defect. Patients with fistulas arising as a result of an obstetrical injury should be routinely evaluated for anatomic sphincter defects. Rectal surgery has often been associated with RVFs. Iatrogenic fistulas are reported in up to 10 % of low rectal anastomoses [7, 8]. A risk factor appears to be the use of double stapling technique [7, 9, 10]. The use of pre- or postoperative external beam radiation plays a role in fistula development and impairs healing [11].
A spontaneous healing is very rare with the exception of Crohn’s disease (CD) more recently with the use of anti-tumor necrosis factor (TNF) therapy, and surgical treatment is usually indicated due to the impact on the quality of life. The choice of the surgical approach is controversial and the results of surgical approaches are highly variable. Furthermore, the majority of published data on RVF pertains to CD [12–16].
The surgical approaches available are numerous, and they vary based on the etiology, location, size, quality of the surrounding tissue, and previous attempted repairs. Surgical approaches can be classified as either local or transabdominal. Local repairs are most useful for low to middle RVFs and include transanal, transvaginal, and perineal approaches. Abdominal operations are usually reserved for high RVFs and may incorporate laparoscopy. The use of healthy muscle or vascularized tissue for transposition is often recommended.
General Principles
Timing is an important part of the surgical decision-making process. In the face of infection or inflammation, it is critical to allow resolution prior to repair. Antibiotic therapy, anti-TNF, or immunosuppressive medications (in case of CD) play an important role in surgical optimization. While a recommended period of 3–6 months on medical therapy has been suggested, surgery should proceed only when surrounding tissues appear reasonably healthy. The use of fecal diversion in preparation to definitive repair or as an adjunct to the repair is also highly controversial and often reserved for recurrent cases after failed surgical treatment, in the presence of CD or after radiation. Preoperatively, the patient undergoes mechanical bowel preparation and receives antibiotics. Procedures may be performed under local anesthetic with sedation, but spinal or general anesthesia is typically preferred. Patients are positioned based on the approach: i.e., for a vaginal approach the patient is placed in a lithotomy position versus prone jackknife position for a transanal approach with exposure facilitated by taping the buttocks or using a Lone Star retractor. The anal canal and vagina are prepared with povidone-iodine solution and a urinary catheter is placed. Patients who require abdominal procedures are placed in the lithotomy position.
Local Repair
Mucosal Advancement Flap Repair
Advancement flaps are the most popular transanal procedure among colorectal surgeons. Many variations exist; however, the general principle remains the same: excision and closure of the rectal portion of the fistula and coverage with a vascularized mucosal flap on the high-pressure side of the fistula. The tract is identified by palpation and probing. The fistula tract is debrided and excised. A flap is created that includes mucosa, submucosa, and muscle placed over re-approximated rectovaginal septum (RVS). The flap base should be at least 2–3 times the width of the apex to ensure adequate vascular supply. The flap mobilization should continue 4–5 cm cephalad to the fistula defect. These principles ensure a tensionless suture line (Figs. 37.2 and 37.3). Success rates vary from 41 to 96 % (Table 37.1) [3, 17–22]. This wide discrepancy may be explained by differences in technique as well as patient selection. Complications are minor and infectious/ischemic in nature. In patients reporting associated incontinence, which is usually secondary to injury to the sphincter mechanism, a sphincteroplasty can be concurrently performed; thus, both correcting the underlying sphincter defect and interposing vascularized muscle in the RVS and perineum.
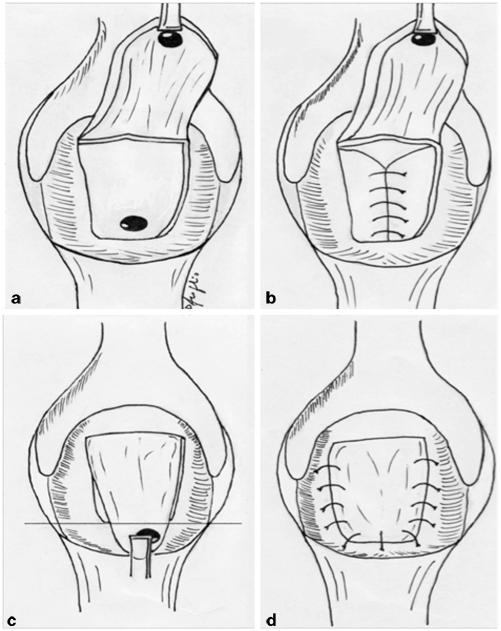
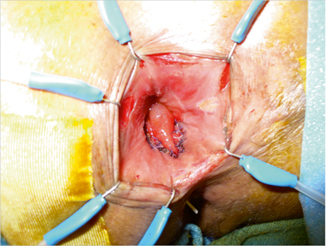

Fig. 37.2
Mucosal advancement flap technique. a A flap is created that includes mucosa, submucosa, and muscular layer; bcurettage and closure of the internal opening; c the distal part of the flap including the rectal opening is excited; dthe flap is advanced to close the defect without tension. (Courtesy of Dr. Daniele Scoglio)

Fig. 37.3
Mucosal advancement flap; intraoperative picture.The well-vascularized broad-based flap covers the anal opening of the rectovaginal fistula
Endorectal Advancement Flap with Muscular Plication (Anterior Levatorplasty)
A similar approach is the advancement flap with plication of the muscular layer. An anterior transverse incision is made distal to the internal opening extending to the submucosa, and a U-shaped flap consisting of mucosa and submucosa is prepared. The dissection is carried out in a cephalad direction until the entire flap can be easily advanced distally. The distal part of the flap, including the internal opening, is then excised. The remaining track is curetted and the internal opening is closed with a figure-of-eight stitch using a reabsorbable suture (3.0 Vicryl). A transverse plication of the muscular layer, internal anal sphincter and/or rectal muscular layer depending on the height of the fistula is made using an absorbable running suture. Finally, the mucosal–submucosal flap is advanced to cover the muscular plication and closed without tension with interrupted absorbable. This approach can be used to treat RVFs without an anal sphincter defect. The goal is to create a second layer of well-vascularized tissue, incorporating healthy tissue under the flap. With this technique, de Parades et al. [23] reported a success rate of 65 %.
Transanal Sleeve Advancement Flap
This technique was described for the first time by Hull and Fazio in 1997 [24] to treat anovaginal fistula in patients with mild Crohn’s proctitis. It is an invasive procedure that involves mobilization and resection of the distal rectum. Re-anastomosis, usually via a transanal manual suture, is performed following the removal of the fistula-bearing area. The procedure is primarily used in patients with significant rectal-wall defects due to chronic inflammatory bowel disease or following radiation therapy. In their study, Hull and Fazio performed five sleeve advancement flaps. Three of the five patients had stomas for fecal diversion. Two stomas were closed without recurrence and the third patient had a recurrence, then a repeat sleeve advancement flap before a successful stoma closure. Of the two patients without fecal diversion, one went on to have a total proctocolectomy.
Transvaginal Repair
Transvaginal (TV) approach is suitable for small low RVF. The vaginal mucosa is incised around the fistula ostium, and the fistula is closed with sutures imbricating the soft tissue towards the anorectum. The vaginal mucosa is then re-approximated. Rahman et al. [25] described their results in 39 patients undergoing TV repair for low RVF and reported a 100 % success rate with this approach. This is a particularly appealing approach in patients with CD as dissection in the diseased rectum can be avoided. Bauer et al. [26] reported their results for 13 patients with CD who underwent repair of RVF by a TV approach. All patients had a diverting intestinal stoma either as part of the initial step in the staged management of intractable perianal disease or concurrent with the repair of the RVF. Each of the patients had low or middle septal fistulas. Fistulas were eradicated in 12 of the 13 women and did not recur during the follow-up period, which averaged 50 months (9–68 months).
Fistulotomy
The use of fistulotomy to treat RVFs is associated with a prohibitive rate of fecal incontinence and is mentioned only to discourage its application.
Ligation of Intersphincteric Fistula Tract
A recently popularized surgical treatment for fistula in ano has been adapted to treat RVF. The ligation of intersphincteric fistula tract (LIFT) involves dissection in a bloodless plane between the internal and external anal sphincters beyond the fistula tract. The tract is then ligated and closed on both the rectal and perianal side. The intersphincteric dissection is then closed at the skin. High success rates after LIFT treatment of fistula in ano are encouraging (60–94 %) [27, 28], but experience with LIFT treatment of RVF is still limited.
Biological Agents: Fibrin Glue and Fistula Plug
Although there have been various reports of successful outcomes in treating anorectal fistulas with biological agents such as fibrin glue [29] and fistula plug [30], the literature is limited to small series. In one small study, four of five patients with RVFs treated with fibrin glue were healed [31]. In different series of reports by Loungnarath et al. [32] ,there was one successful outcome in three patients treated with fibrin glue for RVF. A commonly used type of bioprosthetic fistula plug is made from porcine intestinal submucosa. It is placed through the RVF tract and it is trimmed at both the rectal and vaginal ends when it exceeds the length of the fistula. The plug is then secured with absorbable sutures in a figure-of-eight fashion on the rectal side and the vaginal side is left open for drainage. Experience with this technique in patients with RVFs is limited [33]. Trials that compare rectal mucosal flap advancement to bioprosthetic plug placement for the treatment of fistula in ano are ongoing [34]. Smaller studies show that bioprosthetic plugs are more successful in the treatment of simple anorectal fistulas compared with the complicated ones [35]. Recent modifications to the bioprosthetic to accommodate anatomic features of a RVF may make this approach more successful [36]; however, additional experience is needed to determine the efficacy of bioprosthetics in the use of RVF treatment.
Miscellaneous
The use of autologous stem cells to treat RVFs [37], as well as circular stapler, which has only been published in one case report, are other two options to treat RVFs [38]. Furthermore D’Ambrosio et al. [39] reported the first case series for the treatment of RVFs by transanal endoscopic microsurgery and Lamazza et al. [40] suggested the use of endoscopic-covered stent to treat patients with RVFs and fecal diversion.
Tissue Transfer Procedures
The purpose of tissue transfer procedures in patients with RVFs is to provide healthy, tension free, well-vascularized tissue to support the repair. A multitude of tissue transfers are described including the gracilis, rectus, gluteus, and bulbocavernosus muscles [41–45]. We describe the two most widely used techniques.
Gracilis Muscle Interposition Flap
The gracilis muscle is mobilized based on the proximal major pedicle of the medial femoral circumflex artery after ligation of the distal non-dominant vascular pedicle. A subcutaneous tunnel is created between the thigh incision and the perineum, and the distal end of the muscle is tunneled under the skin to the perineal wound. The gracilis is interposed between the rectum and vagina without tension after the fistula is closed. The proximal end of the muscle is tunneled between the rectum and vagina and tacked 3 cm above the suture lines of both the rectal and vaginal defects and down to the opening of the perineal wound. Meticulous hemostasis is achieved. The thigh and perineal wounds are closed primarily after placing drains. The gracilis muscle is an excellent option,because it is a functionally rudimentary muscle, and thus expendable without noticeable functional deficits. Furthermore, it is easily mobilized with adequate length, and has a dominant vascular pedicle proximally that is convenient for perineal transposition allowing transfer of the distal end to the upper RVS without tension on its vascular pedicle. Several studies have shown high success rates as when the gracilis is used to close RVFs [41, 46, 47].
Zmoraet al. [41] reported their experience with gracilis muscle interposition. The authors included five patients with a RVF and one patient with a pouch-vaginal fistula who underwent this repair with favorable results. All patients had fecal diversion as a step preliminary to or concurrent with fistula repair. Five of the six repairs healed completely after the reversal of the fecal diversion. One patient with severe crohn’sproctitis failed and had a persistent RVF.
Martius Flap
The principles of repair involve transposing a pedicle graft harvested from the labia majora through a subcutaneous tunnel [48]. The graft overlies the rectal closure and separates the rectal and vaginal walls, filling in the dead space and stimulating tissue growth and healing. Patients with uncontrolled perineal sepsis or severe fecal soiling should undergo fecal diversion. Repair of the fistula should not be attempted until perineal sepsis and inflammation resolves. A vertical incision is made in the perineum or in the posterior vaginal wall (Fig. 37.4) and is carried out to the inferior margin of the fistula. Local anesthetic is injected into the RVS for hemostasis and tissue dissection. The posterior vaginal wall is sharply mobilized from the rectum. Wide mobilization of the rectum and vagina is necessary so that a multilayer closure can be performed, and re-approximation of the tissue surfaces can occur without any tension. Local anesthetic is injected into the labia majora. A vertical incision is made in the labia majora to expose the bulbocavernosus fat pad. The borders of dissection include the labial crural fold laterally, the labia minora and the bulbocavernosus muscle medially, and the Colles’ fascia covering the urogenital diaphragm posteriorly. A flap harvest is accomplished in a lateral to medial fashion. For RVF repair, the blood supply to the graft is based on the posterior vessels, which includes the perineal branch of the pudendal artery. The entire thickness of the fibro adipose flap is included in a small Penrose drain. Gentle downward traction is applied to aid in the dissection. The graft is transected superiorly. The operator should not divide the pedicle graft until it has been determined that adequate length has been developed. A hemostat is then used to transfer the fibro adipose pad from the harvest site, through the tunnel, to the level of the fistula repair. It’s very important not to twist the graft, and to ensure that it is properly oriented. The fistula tract is excised. The vaginal wall is re-approximated with reabsorbable sutures. This should be a tension free repair. The rectal edges are also freshened up and the rectal mucosa is approximated with absorbable sutures. The flap sits between the rectum and the vagina. The sphincter muscles are re-approximated. The flap is gently sutured into position. Hemostasis is obtained, the wound is irrigated, and the perineal skin is then closed. A small drain is left to keep the wound open. The labial skin is closed in two layers with absorbable sutures. A Penrose drain is left at the inferior border of the incision for drainage. Success rates range from 60 to 100 % [44, 45, 49–52].
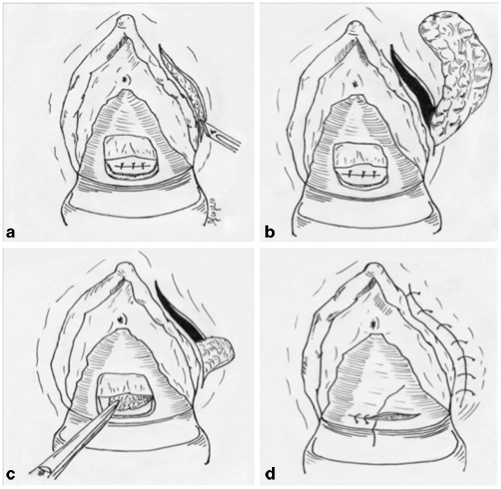

Fig. 37.4
Martius flap technique. a Curved incision of the posterior vaginal wall and suture of the fistula; vertical incision in the labia majora to expose the bulbocavernosus fat pad, b exposition of the fibro-adipose pad, c the pad is transferred from the harvest site, through the tunnel, to the level of the fistula repair, d final suture of the vaginal wall and the labia majora. (Courtesy of Dr. Daniele Scoglio)
Kin et al. [48] reported a series of five patients with a mean age of 48.4 years (range 32–64). Etiologies of the fistulas were: obstetric, iatrogenic (after hysterectomy), CD, cryptoglandular, and idiopathic. The patients had undergone a mean of 2.6 (range 1–5) prior repairs. Of the total of 13 prior attempted repairs, eight were advancement flaps, two were episio-proctotomies, two were fistula plugs, and one was an interposition mesh graft. Three of the five had diverting ileostomies prior to the Martius flap procedure; one underwent diverting ileostomy at the time of the Martius flap procedure. The time from the first symptoms to the first attempted repair was a mean of 14.4 months (range 2–31 months). All repairs involved either sphincteroplasty or perineoplasty in addition to the flap repair. Mean follow-up was 25.6 months (range 3–44). There were no cases of wound complications, recurrence, or functional complications such as dyspareunia. Three of the four patients who had undergone diverting ileostomy have undergone ileostomy reversal. Patients often have associated asymptomatic sphincter defects that should be repaired at the time of fistula repair.
White et al. [44] performed 14 Martius procedures on 12 patients with radiation-induced RVFs. Eleven patients had successful closure of their fistulas with this procedure, and no operative complications occurred. Aartsen and Sindram [45] reported results in 20 patients with radiation-induced RVF. In this study, nine procedures were done without and 14 procedures with a Martius flap. After a mean follow-up of around 10 years, the success rate of fistula repair was 5 of 9 (55 %) and 13 of 14 (93 %), respectively.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








