(1)
Middlemore Hospital, Department of Surgery, University of Auckland, Auckland, New Zealand
Keywords
Presacral bleedingPresacral venous plexusBasivertebral veinsRectal surgerySuture ligationThumbtacksHaemostatic agentsIntroduction
Presacral or pelvic bleeding is a rare but potentially catastrophic intraoperative surgical emergency, which may be encountered during rectal dissection. It is characterised by high-volume bleeding, which is difficult to control with conventional means and can lead rapidly to hypovolaemic shock and death. The reported incidence varies from 4.6to9.4 % in open surgery, and it is likely that the incidence is equivalent during laparoscopic and robotic resection [1, 2]. Even in high-volume institutions, this incidence may equate to an individual surgeon dealing with significant pelvic bleeding as infrequently as once every year. The uncommon nature of this problem, however, makes it imperative that all surgeons who operate in the pelvis, particularly those who may not do so regularly, understand the basis, significance and prompt management of this problem and formulate an individualised plan in line with personal preference and availability of necessary aids within their institution (Fig. 38.1).
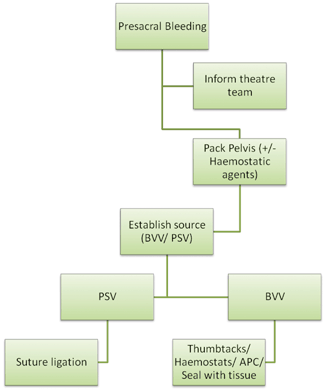

Fig. 38.1
Algorithm for management of presacral bleeding. PSV presacral veins, BVV basivertebral veins, APC argon plasma coagulation. Note that the techniques used for BVV bleeding can also be used for PSV bleeding
Anatomy
The vascular anatomy of the pelvis is variable. Cadaveric studies have demonstrated inconsistent anatomical variations even when studying relatively small samples [3]. Significant bleeding, however, occurs from either the presacral venous plexus or the basivertebral veins. The two are linked and provide a connection between the inferior vena cava and the vertebral venous system. Vascular injury results in pronounced bleeding since the veins are part of an avalvular system. The veins are intrinsically friable due to their low-pressure, high-capacitance characteristics. Moreover, since patients are often in the modified Lloyd-Davis position for access to the pelvis, the distal presacral veins that are most vulnerable to injury lie in the lowest position and may have 2–3 times higher hydrostatic pressure than the inferior vena cava [4]. During in vitro experiments, the rate of bleeding from a vein 2–4 mm in diameter has been shown to be over 1 l/min [4].
The presacral venous plexus is formed by the middle sacral, lateral sacral and communicating veins and is the distal continuation of the anterior branches of the external vertebral venous plexus. The basivertebral veins penetrate sacral foramina from S3 to S5 and penetrate through the spongiosa of the sacral bone via a series of canals acting as a venous sinus [4]. The intrasacral canal venous plexus can be considered to be a terminal part of the vertebral venous system, thereby explaining the massive bleeding seen upon injury. Since the adventitia of the veins is blended to sacral periosteum at the foraminal opening, when the veins are lacerated during dissection, they retract into the sacral foramen. The basivertebral veins end in the presacral venous plexus anteriorly.
Patterns of Injury
As mentioned above, the key anatomical structures that contribute to significant bleeding lie posteriorly in the pelvis. Thus, a breach in the presacral fascia increases the risk of injury to venous structures with consequent bleeding. Therefore, although anterior rectal mobilisation can be more technically challenging, the risk of bleeding is greatest during posterior rectal dissection.
An oncologically sound operation requires dissection between the fascia propria of the rectum and the presacral fascia to ensure a total mesorectal excision (TME) [5]. However, this can be difficult for a number of reasons. It can be difficult to visualise the correct anatomical plane in patients who have had preoperative radiation or previous pelvic surgery with secondary adhesions. Recurrent or advanced malignancy can pose similar problems. Obese patients or those with a narrow pelvis can pose difficulties in achieving optimal access. Moreover, for surgeons beginning to perform laparoscopic or robotic TME, the learning curve may also lead to inaccuracies in dissection. A higher rate of intraoperative bleeding has been demonstrated in the context of laparoscopic colonic resection, and previous reports have also suggested that surgeon’s inexperience may contribute to an increased risk of pelvic bleeding [2, 6].
Qinyao et al. have demonstrated the patterns of injury encountered during rectal dissection [4]. This includes the now largely abandoned practice of blunt mobilisation of the rectum posteriorly. Other reasons include laceration of the presacral fascia or clamping bleeding vessels on the presacral fascia and avulsing them with or without periosteum. The authors make a specific distinction between injury to the presacral venous plexus or to basivertebral veins [4].
It is important to acknowledge that increasing surgical intervention for advanced or locally recurrent pelvic malignancies has led to more radical and en bloc, non-anatomical resections. These operations are of longer duration and are characterised by greater blood loss in general and resections including sacrectomy, or pelvic sidewall dissections may involve high-volume, brisk bleeding due to non-traditional patterns of injury [7]. Nonetheless, the principles of management remain the same.
Management
The likelihood of encountering significant pelvic bleeding is highest in a patient with numerous unfavourable characteristics (obese, narrow pelvis, advanced malignancy). Thus, the operation is likely to be difficult to begin with, and it is likely that by the time bleeding is encountered, the surgeon may already be physically tired and stressed. The literature has consistently demonstrated that checklists improve outcomes in a stressful situation [8]. As such, it is important to have a structured plan.
Prior to engaging in specific technical manoeuvres described below, the situation first needs to be acknowledged. Troublesome bleeding in the pelvis is a constant hindrance but does not impede progress nor compromise the patient. Significant bleeding, however, should be verbally acknowledged. This is important since all members in operating room may not be able to see the operation or may not be aware of the gravity of the situation. It is possible that the fellow or resident either performing or assisting in the operation may not have encountered significant pelvic haemorrhage before [9]. It will thus be necessary for the surgeon to take over the operation and organise the assistants in the most useful position (e.g. opposite the surgeon or in between the patient’s legs) as per their seniority. An escalation in hierarchy should also be conducted with other members of staff in theatre. The most senior nurse should take over as the scrub nurse if not already involved, and multiple unscrubbed nurses should be available as a number of uncommonly used tools may need to be acquired expediently. Similarly, the anaesthesiologist should be informed directly.
Role of the Anaesthesiologist
Once the anaesthesiologist has been informed, acute circulatory support can be considered to have been delegated. Though the practical role of the surgeon is to control the bleeding, it is important to be familiar with the strategies at the disposal of the anaesthesiologist.
The focus of the anaesthesiologist will include initial measures including ensuring optimal intravenous access and volume resuscitation. This may lead to more intensive monitoring for the acute period and also in anticipation of the likely necessity of admission to the high dependency unit or intensive care unit.
Volume replacement may include activation of the massive transfusion protocol or component blood replacement in line with institutional practice. Serial thromboelastograph measurements may also be important to guide blood product replacement alongside advice from a haematologist [10].
Tranexamic acid may also help limit the magnitude of bleeding. Recent evidence suggests that tranexamic acid reduces bleeding in gastrointestinal surgery with no increase in the incidence of thromboembolic events [11]. Anecdotal experience also suggests that use during pelvic exenteration decreases surgical ‘ooze’.
Role of the Surgeon
Once the operating team has been suitably organised, the pelvis should be packed. At this time, more help should be obtained from a colleague if possible. The importance of an experienced first assistant and the potential advantage of a hitherto uninvolved person cannot be overstated.
The injury now needs to be localised and illumination and proper exposure is crucial especially since a hallmark of this type of bleeding is gushing blood form the pelvic floor with a near-undetectable bleeding point. A practical solution to obtain optimal illumination is that the operating surgeon should utilise a headlight. Exposure can also be facilitated by expedient removal of the specimen whilst the area remains tamponaded with packs. If the bleeding point cannot be reliably detected, pressure should be maintained for 15–20 min. The temptation to re-examine the area ahead of this interval should be resisted, and the time should be used to formulate a plan and obtain necessary equipment. The pressure effects of packing may also be complemented with commercially available fibrin-based haemostatic agents [12]. A combination of haemostatic matrices (e.g. FloSeal, Baxter, USA) and absorbable haemostatic products (e.g. Surgicel Fibrillar, Ethicon, USA) may be used.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








