Management of Lipid Abnormalities in the Patient with Kidney Disease
Christoph Wanner
Vera Krane
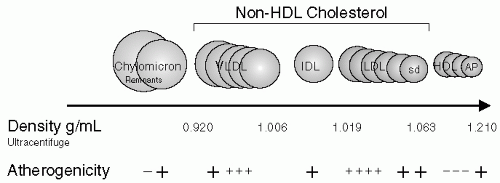
To understand dyslipidemia in chronic kidney disease (CKD) stages 1 through 5, knowing some general aspects of lipid metabolism is useful. In general, all five major lipoprotein classes (chylomicrons, very low-density lipoproteins [VLDL], intermediate-density lipoproteins [IDL], low-density lipoproteins [LDL], high-density lipoproteins [HDL]) consist of lipids (cholesterol, triglycerides, and phospholipids) and apolipoproteins. Apolipoproteins (A-I, A-II, B-48, B-100, C-I, C-II, C-III, and E) are found in different distributions among the various lipoproteins and serve as cofactors for enzymes and ligands for receptors. Levels of chylomicrons, the largest lipoprotein particle, increase after eating and are almost absent in the fasting state. These lipoproteins are formed in the intestinal epithelial cells; their main lipid content, triglycerides, is synthesized from re-esterification of dietary monoglycerides and fatty acids. Triglycerides represent 90% of chylomicrons and are hydrolyzed by lipoprotein lipase (LPL) present in adipose and vascular tissue. The residual particles, also called chylomicron remnants, are usually removed rapidly by the liver. In addition to dietary-derived chylomicrons, the liver has the capacity to produce endogenous lipoproteins from excess hepatocyte cholesterol and triglycerides. These lipids are synthesized and are secreted as triglyceride-rich VLDL. The triglycerides present in VLDL are gradually removed by LPL (with apoC-II acting as a cofactor), resulting in IDL. IDL, also named VLDL remnants, represents a transition step in the lipolysis of VLDL to LDL, the main cholesterol-carrying lipoprotein, which accounts for 70% of circulating cholesterol (Fig. 4-1). The characteristics of the various lipoproteins and their lipid and apolipoprotein composition in healthy humans are given in Figure 4-1.
Lipoprotein(a) [Lp(a)], another apolipoprotein, should not be neglected in patients with kidney disease, because high levels are especially atherogenic. Lp(a) contains a structural protein called [apo(a)]. Apo(a) exhibits a high homology to plasminogen and an extreme size polymorphism, with the apo(a) isoproteins ranging in size from 420 to 840 kD. Inherited in an autosomal-codominant fashion, the apo(a) isoprotein is an important factor that determines plasma Lp(a) concentrations, with an inverse correlation between the size of apo(a) isoprotein and the plasma Lp(a) concentration. The distribution of plasma Lp(a) levels is highly skewed toward lower concentrations, with more than two thirds of the population having levels lower than 20 mg per dL. High plasma concentrations of Lp(a) (more than 20 mg per dL) are associated with the risk for premature coronary atherosclerosis, cerebrovascular atherosclerosis, and saphenous vein bypass graft stenosis.
TYPES OF DYSLIPIDEMIA IN DIFFERENT STAGES OF CHRONIC KIDNEY DISEASE AND RENAL REPLACEMENT THERAPY
General Aspects
Qualitative characteristics of dyslipoproteinemia are similar in early renal insufficiency and in advanced kidney failure. The main metabolic abnormality is hypertriglyceridemia and delayed catabolism of triglyceride-rich lipoproteins resulting in increased concentrations of very low density lipoproteins (VLDLs) and intermediate density lipoproteins (IDLs) and reduced levels of HDL. Plasma cholesterol concentration is usually normal, even reduced, and only occasionally elevated. Dyslipidemia is detected in an early stage of chronic kidney disease (CKD, stage 2) when diagnosed and characterized by abnormalities in the composition of apolipoproteins. Increased levels of apoC-III and decreased levels of the apoA-I/apoC-III ratio are considered to be the hallmarks of an altered profile in kidney disease.
Chronic Kidney Disease and Proteinuria: Impact on Serum Lipids and Lipoproteins
Dyslipidemia is present in 70% to 90% of patients with nephrotic syndrome and most often expressed as both an increase in the serum total cholesterol and/or LDL cholesterol and increased serum triglycerides (50%). One third of patients have an exclusive elevation of LDL cholesterol, whereas only 4% of patients show pure hypertriglyceridemia. Changes in the composition of lipoprotein particles also have been described, with cholesterol enrichment in IDL but not LDL. The levels of HDL cholesterol may vary. This could be because of high serum lipoprotein(a) [Lp(a)] contaminating the HDL samples when cholesterol is assayed. The reasons why patients with nephrotic syndrome present different accumulations of triglycerides and cholesterol may include factors such as genetic apolipoprotein phenotypes, concomitant drug therapy, and the catabolic state of the individual. Delayed catabolism and over synthesis of lipoproteins are operative. Two separate processes impede the removal of triglyceride-rich lipoproteins
in nephrotic syndrome. One is an abnormality in VLDL that decreases the ability to bind to endothelial surfaces in the presence of saturating LDL. This defect in VLDL function, and presumably structure, results from proteinuria. The second defect is the inability of LDL to bind effectively to vascular endothelium. Whereas VLDL levels are high because of reduced catabolism, LDL levels are increased because of increased synthesis. The presence of uremia in patients with nephrotic syndrome leads to further changes. Markedly elevated Lp(a) concentrations have been found in the majority of patients with proteinuria and nephrotic syndrome and resolve when remission of the nephrotic syndrome is induced. There are data to suggest that increased synthesis, rather than decreased catabolism, causes elevated plasma Lp(a) concentrations in nephrotic syndrome.
in nephrotic syndrome. One is an abnormality in VLDL that decreases the ability to bind to endothelial surfaces in the presence of saturating LDL. This defect in VLDL function, and presumably structure, results from proteinuria. The second defect is the inability of LDL to bind effectively to vascular endothelium. Whereas VLDL levels are high because of reduced catabolism, LDL levels are increased because of increased synthesis. The presence of uremia in patients with nephrotic syndrome leads to further changes. Markedly elevated Lp(a) concentrations have been found in the majority of patients with proteinuria and nephrotic syndrome and resolve when remission of the nephrotic syndrome is induced. There are data to suggest that increased synthesis, rather than decreased catabolism, causes elevated plasma Lp(a) concentrations in nephrotic syndrome.
Hemodialysis and Dyslipidemia
Dyslipidemia in patients undergoing hemodialysis is more frequent than in the general population and is characterized by hypertriglyceridemia and low levels of HDL. Levels of total cholesterol and LDL usually are normal. This translates into the most characteristic feature of the end-stage renal disease (ESRD)-associated dyslipidemia represented by an accumulation of triglyceride-rich lipoproteins (VLDL remnants) and IDL. Catabolism of IDL and LDL is severely impaired, resulting in a markedly prolonged residence time of both particles. Additionally, qualitative changes of LDL with formation of small dense LDL are found. By the level of apolipoproteins, the dyslipidemia can also be classified as an accumulation of apoB-containing triglyceride-rich lipoprotein particles containing apoC-III and Lp(a) or lipoprotein B complex particles.
Besides a defect in postprandial chylomicron remnant clearance, abnormal HDL apoA-I and apoA-II kinetics have been described, with increased catabolism of apoA-I and decreased production rate of apoA-II resulting in reduced plasma levels of both apolipoproteins.
Continuous Ambulatory Peritoneal Dialysis and Dyslipidemia
Patients undergoing continuous ambulatory peritoneal dialysis (CAPD) present with higher plasma cholesterol, triglyceride, LDL, and Lp(a) levels than patients undergoing hemodialysis. This additional increase is most likely because of two factors: (i) loss of protein (7-14 g/day) into the peritoneal dialysate, mimicking nephrotic syndrome, and (ii) absorption of glucose (150-200 g/day) from the dialysis fluid. This was in part reflected in the data from Prinsen et al., who reported that VLDL-1 apoB100 and VLDL-2 apoB100 pool sizes were increased because of disturbances in both synthesis and catabolism. VLDL-1 apoB100 production is at least partially explained by increased free fatty acid availability secondary to peripheral insulin resistance; thus, insulin resistance might be a potential therapeutic target in patients undergoing peritoneal dialysis. In general, qualitative lipoprotein abnormalities are similar to those found in patients undergoing hemodialysis, and most mechanisms altering lipoprotein metabolism are probably also qualitatively the same, even
though a study in normolipidemic CAPD patients demonstrated less pronounced abnormalities of cholesterol transport than observed in patients undergoing hemodialysis.
though a study in normolipidemic CAPD patients demonstrated less pronounced abnormalities of cholesterol transport than observed in patients undergoing hemodialysis.
Lipid Abnormalities after Kidney Transplantation
Posttransplant dyslipidemia is qualitatively and quantitatively dependent on age, gender, body weight and type, and dose of immunosuppressive agents. The prevalence of lipid changes in kidney transplant recipients is very high. Particularly common are increases in cholesterol and LDL. HDL is usually normal, and triglycerides are often increased.
DYSLIPIDEMIA AND IMPACT ON CARDIOVASCULAR DISEASE
The overall risks of cardiovascular morbidity and mortality are profoundly increased in patients with CKD, and the majority of patients with CKD die of cardiac and vascular events before reaching ESRD.
Nephrotic Syndrome
There is no reason to doubt that the severe, persistent elevations of cholesterol, LDL, IDL, and Lp(a) do not represent a highly atherogenic condition. However, relatively little and conflicting information has been published on the risk of atherosclerotic vascular disease in patients with CKD. All of these studies were retrospective, however, and flawed by small sample numbers, selection bias, and lack of control for other atherosclerotic risk factors. Some studies included patients with minimal change disease, and most of these patients would most likely resolve their nephrotic syndrome and hence not remain at risk for the long-term complications of hyperlipidemia. According to Ordonez et al., the adjusted relative risks of myocardial infarction and coronary death in nephrotic syndrome are 5.5 and 2.8, respectively. Data were obtained for 142 patients matched with healthy controls and followed prospectively for 5.6 and 1.2 years.
Dialysis
Cardiac and vascular disease is the leading cause of morbidity and mortality in patients undergoing hemodialysis; cardiac disease accounts for 44% of overall mortality. Approximately 22% of these deaths from cardiac causes are attributed to acute myocardial infarction but are only 10% of all-cause mortality. In patients who survive a myocardial infarction, the mortality from cardiac causes is 59% at 1 year, 73% at 2 years, and nearly 90% at 3 years. After adjusting for age, gender, race, and diagnosis of diabetes mellitus, mortality from cardiovascular disease (CVD) is far greater in patients undergoing hemodialysis than in the general population. It ranges from 500-fold in individuals aged 25 to 35 years to fivefold in individuals aged >85 years. This might be because of an increased prevalence of traditional and kidney disease-related risk factors. In this context, lipid abnormalities have been suggested as a major cause of vascular disease in hemodialysis patients. Whereas the log-linear relation between risk of coronary artery disease and blood cholesterol is well-established in the general population, most cross-sectional studies




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree