Description
Treatment
Special considerations
Anal Intraepithelial Neoplasia (AIN)
Dysplasia of squamous cells of the anal margin/canal; precursor lesion of SCC
Surveillance for low-grade lesions
Common in HIV +
HIV treatment can influence progression to SCC
Low-grade (AIN I)
Topical treatment with Imiquimod or 80 % trichloroacetic acid
Also common in HIV- homosexual men
Moderate-grade (AIN II)
Cryotherapy
High-grade (AIN III)
Bowen’s Disease
Squamous cell carcinoma in situ of the anal margin (high-grade dysplasia)
Unifocal disease: local excision/ablation
Modern treatment approaches converging with management of AIN
Multifocal disease: topical treatment with Imiquimod or 80 % trichloroacetic acid
Buschke-Lowenstein tumor
Intermediate lesion between condyloma and invasive SCC; can be very large (up to 30 cm)
Wide-local excision may require APR if there is extensive sphincter involvement
Paget’s disease
Adenocarcinoma in situ of the anal margin; 50–70 % association with other lower GI malignancies
Wide local excision/ablation
Colonoscopy to evaluate for other lower GI lesions
51.5 Anal Margin Malignancies
The vast majority of anal margin cancers are squamous cell in origin. They are similar to SCC of other areas of the body, and, thus, have a similar staging system and treatment. Small tumors that are superficial, such as T1 lesions, may be eligible for treatment with wide local excision. More advanced tumors are typically treated with combined modality chemoradiation therapy regimens. Those that fail this treatment may benefit from surgical therapy. 5-year survival rates range from 70 to 90 % [21].
Basal cell carcinoma of the anal margin is very rare. Like basal cell carcinoma of other parts of the body, wide local excision is the treatment of choice. This tumor often recurs, and re-excision is needed. 5-year survival is nearly 100 % [21].
Perineal Coverage After Resection of Anal Margin Tumors
When wide local excision is performed in the treatment of anal margin tumors, a large defect may remain, and consideration needs to be given to the method of reconstruction of the perineal wound. Split-thickness skin grafts can be used for coverage, especially for superficial wounds [29, 30]. Most cases, however, involve deep wounds, and STSG will result in large defects and poor cosmesis for the patients. Local flaps, such as the V-Y gluteal advancement flap, are commonly used, with generally good results but an approximately 30 % wound dehiscence rate [31].
Rotational myocutaneous flaps, based on a vascular pedicle, can also be used. The gracilis and, when associated with abdominoperineal resection, rectus abdominis flaps are most commonly employed. These flaps can be especially useful in patients with prior pelvic irradiation or those who have failed primary closure of large defects [32, 33].
51.6 Anal Canal Tumors
There are many different types of anal canal tumors, including adenocarcinoma, melanoma, GIST, and epidermoid carcinoma. The histology of epidermoid carcinoma includes squamous cell, basaloid, cloacogenic/transitional cell, and mucoepidermoid types. Squamous cell carcinoma is by far the most common, comprising nearly 80 % of these tumors, but the histologic phenotype may be mixed [34, 35].
Diagnosis
The key to anal cancer management is early diagnosis. The most common presenting complaint is bleeding, occurring in >50 % of patients. Patients may also complain of anal pain, pruritus, tenesmus, change in bowel habits, abnormal discharge, or the sensation of a mass. These complaints are also associated with benign anorectal conditions, and it is important for the clinician to avoid misdiagnosis of anal cancer [34, 35].
Physical examination is important in diagnosis and can help in staging (Table 51.2). The size of the tumor, its appearance, and fixity to surrounding organs or the bony pelvis are important components of preoperative evaluation, as anal SCC often presents at a locally advanced stage. The most common finding is an intraluminal mass that may be exophytic, ulcerated, or flat. On digital rectal exam, anal cancer could be mistaken for a hemorrhoid, so it is important to visualize the lesion with anoscopy/proctoscopy. Colonoscopy is also essential for ruling out more proximal malignancies. For diagnosis, incisional biopsy is recommended. Palpation for inguinal lymphadenopathy should be performed, as approximately one third of anal SCC will demonstrate regional lymph node metastases. Palpable inguinal lymph nodes can be sampled via fine needle aspiration. Sentinel lymph node biopsy has also been investigated, but patients with negative results can later present with inguino-femoral nodal disease [36].
Table 51.2
Staging of anal canal tumors
Stage | T | N | M |
|---|---|---|---|
0 | Tis | N0 | M0 |
I | T1 | N0 | M0 |
II | T2 | N0 | M0 |
T3 | N0 | M0 | |
IIIA | T1 | N1 | M0 |
T2 | N1 | M0 | |
T3 | N1 | M0 | |
T4 | N0 | M0 | |
IIIB | T4 | N1 | M0 |
Any T | N2 | M0 | |
Any T | N3 | M0 | |
IV | Any T | Any N | M1 |
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
Tis | Carcinoma in situ (i.e., Bowen disease, high-grade squamous intraepithelial lesion, and anal intraepithelial neoplasia II–III) | ||
T1 | Tumor ≤2 cm in greatest dimension | ||
T2 | Tumor >2 cm but ≤5 cm in greatest dimension | ||
T3 | Tumor >5 cm in greatest dimension | ||
T4 | Tumor of any size invades adjacent organ(s), e.g., vagina, urethra, and bladder | ||
NX | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | Metastases in perirectal lymph node(s) | ||
N2 | Metastases in unilateral internal iliac and/or inguinal lymph node(s) | ||
N3 | Metastases in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes | ||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
The initial workup and clinical stage assessment should include computed tomography of the abdomen and pelvis to evaluate the primary lesion, regional lymph nodes, and to rule out distant metastatic disease [34, 35]. Endorectal ultrasound and MRI are other modalities that can be used for locoregional staging; however, the role for ultrasound may be limited as primary tumor staging is dependent upon tumor size, not depth of penetration in the bowel wall or sphincter complex [37–39]. A chest radiograph or CT of the chest should also be obtained. Fluorodeoxyglucose positron emission tomography with CT has been reported to show utility for staging of anal cancer and may be considered, although its ability to replace conventional contrast-enhanced CT has not been validated [40–45].
51.7 Treatment
Until the 1970s, the primary treatment of anal canal carcinoma was abdominoperineal resection with or without inguinal lymph node dissection. This procedure carried with it high morbidity and had a significant impact on the patient’s overall quality of life, while achieving overall survival of only 40–70 %. In 1974, Nigro observed that many patients with locally advanced disease treated with neoadjuvant chemoradiation followed by APR had a complete pathologic response. This led him and other investigators to develop a protocol of radiation therapy with concurrent chemotherapy with 5-fluorouracil and mitomycin-C, and they found overall survival rates in the range of 65–85 % [46, 47]. This discovery has revolutionized the treatment of anal canal carcinoma, and today the primary treatment is combined modality chemoradiation therapy. Since its development, multiple studies have investigated modifications of the Nigro protocol in order to improve treatment response and reduce toxicity.
Radiation Therapy
In 1996, the UK Coordinating Committee on Cancer Research (UKCCCR) reported results from the ACT I trial [48]. This study evaluated the benefit of combined modality therapy versus radiation therapy alone. Radiotherapy had demonstrated 3-year survival rates as high as 75 %, but local control rates around 40–50 %. In this study 585 patients were randomized to receive a total radiation dose of 45 Gy in 20 or 25 fractions over 4–5 weeks or the same radiation therapy dose with the addition of concurrent chemotherapy with 5-FU (1,000 mg/m2 over 4 days or 750 mg/m2 over 5 days) during the first and final weeks of radiation. Mitomycin C (12 mg/m2) was also given on treatment day 1. After a median follow-up of 42 months, local failures occurred in 59 % of the radiotherapy group versus 36 % in the CMT arm, reflecting a 46 % risk reduction for local failure in the group undergoing CMT. The European Organization for Research and Treatment of Cancer (EORTC) study 22,861 confirmed the findings of ACT I, with 5-year local failure rates of 50 and 32 % for radiotherapy alone and CMT, respectively [49]. Two separate studies, RTOG 92–08 and ECOG 4292, examined radiation dose intensification in an effort to improve local control but did not show any improvement compared to standard therapy [50, 51].
Intensity modulated radiation therapy (IMRT) has shown promise in allowing for an increase in dose while sparing surrounding tissues and decreasing toxicity [52]. In a phase II evaluation, RTOG 05–29 demonstrated a reduction of acute grade 2+ hematologic and grade 3+ dermatologic and GI toxicity with IMRT and concurrent chemotherapy with 5-FU and MMC [53]. Brachytherapy is another strategy to optimize local control while limiting pelvic toxicity, but this approach remains investigational [54, 55].
Cisplatin-Based Chemotherapy Regimen
Despite the efficacy of concurrent 5-FU and MMC chemoradiation therapy, it is associated with significant treatment associated toxicity. Early experience with 5-FU and cisplatin-based chemoradiation therapy suggested improved response rates with reduced toxicity [56–58]. Thus, the UKCCCR ACT II trial compared concurrent chemoradiation therapy regimens with 5-FU and MMC or cisplatin. Additionally, the potential for maintenance chemotherapy to improve survival was assessed. In this 2 × 2 factorial study, 940 patients received either cisplatin-based chemoradiation with (n = 222) or without (n = 246) maintenance chemotherapy or MMC-based chemoradiation therapy with (n = 226) or without (n = 246) maintenance chemotherapy. The complete response rate at 26 weeks was 89.6 % versus 90.5 % for the cisplatin vs. MMC groups, respectively. Furthermore, there were no significant differences in the toxicity profiles between the groups. After a median follow-up of 5.1 years, maintenance chemotherapy was not associated with improved 3-year progression-free survival [59].
Induction Chemotherapy
Based on favorable preliminary outcomes with cisplatin-based induction chemotherapy for patients with advanced cancer and the high rates of toxicity associated with MMC based regimens, the strategy of induction chemotherapy was compared to standard MMC chemoradiation in RTOG 98–11 [60–62]. Six hundred forty-four patients were randomized to receive induction chemotherapy with 5-FU and cisplatin followed by 5-FU and cisplatin chemoradiotherapy or to receive standard 5-FU and mitomycin C based chemoradiotherapy. No significant difference was observed in 5-year disease-free (60 % for MMC vs. 54 % for cisplatin) or overall survival (75 % vs. 70 %). Although the rate of severe hematologic toxicity was greater in the MMC arm, the rate of colostomy after treatment was greater in the induction + cisplatin-based chemoradiation arm (19 % vs. 10 %). Thus, the current MMC-based regimen without induction chemotherapy remains the standard of care for anal canal cancer, although cisplatin-based therapy may be acceptable for patients with severe MMC-related toxicity [61, 62].
51.8 Toxicities Associated with Treatment
There are a number of acute and long-term toxicities associated with combined modality treatment of anal cancer. Short-term toxicities are primarily hematologic, particularly with the use of MMC. Severe late toxicities are observed in 10–15 % of patients and are most commonly related to radiation enteritis/proctitis and skin-associated complications. Other toxicities include radiation cystitis and sacral insufficiency fractures [63–67]. The most common symptom with radiation injury is bleeding, but patients may also complain of diarrhea, urgency, tenesmus, and pain. Patients may also present with fistulas and strictures. Colonoscopy or barium enema should be performed to rule out malignancy as a cause of the patient’s symptoms [66, 67].
The primary treatment of radiation-induced toxicity is non-surgical. For radiation-induced proctitis, steroid retention enemas, endoscopic application of formalin, endoscopic argon plasma coagulation, and hyperbaric oxygen therapy have all been tried with varied success. For stricture, endoscopic dilation can be attempted [66].
In patients who have failed medical treatment or those with stenosis, obstruction, refractory pain, perforation, abscess, or fistula, surgical management may be necessary. Options include diversion or resection with or without restoration of intestinal continuity [66]. The choice of operation is dependent upon the patient’s presenting problem, with proximal strictures responding well with resection and distal strictures or proctitis requiring permanent diversion [67]. It is also imperative to know the patient’s anorectal function in determining the ideal procedure for them.
51.9 Follow-Up After Treatment
As many as 25 % of patients will develop local recurrence within the first 3 years. Patients should be evaluated every 3–6 months for the first 3 years and then every 6–12 months for an additional 2 years. Clinical evaluation with digital rectal exam, palpation of inguinal lymph nodes, and anoscopy is key. Any suspicious lesion should be biopsied to assess for persistence or recurrence of disease. Response to treatment may still be ongoing at 12 weeks after completion of chemoradiation therapy so it is important to correlate post-treatment and pre-treatment findings on clinical examination. Surveillance imaging, such as CT or MRI may help to identify distant or inguinal recurrence; however, the secondary salvage treatment options may be limited.
Patterns of Recurrence
Within 6 months of completion of CRT, evidence of tumor within the anal canal is considered to be persistent disease; whereas, beyond 6 months after complete clinical response, it is recurrent disease. The most common site of recurrence is locoregional—within the anorectal canal, the pelvis, or in the inguinal lymph node basin—and occurs in as much as 30 % of patients. Distant metastatic disease is also seen in as much as 20 % of patients who fail primary therapy. T and N stage of disease have been shown to predict locoregional recurrence, while N stage and basaloid subtype are predictors of distant metastases [68].
51.10 Salvage Therapy for Persistent/Recurrent Disease
Local Recurrence
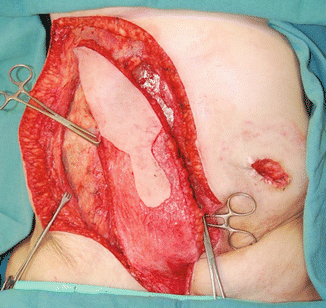
Historically, surgery was the mainstay of therapy in the treatment of anal canal malignancies, but it is now reserved for patients who demonstrate persistent or recurrent disease after definitive chemoradiotherapy. The goal of surgery is to obtain local control and prevent distant recurrence. Surgical resection should be complete and en bloc (R0) to include the perianal skin, involved surrounding organs (e.g., uterus, vagina, prostate), and coccyx or sacrum as the achievement of negative margins is important to prevent locoregional recurrence in these patients (Fig. 51.1). In some cases, pelvic exenteration may be necessary to achieve an R0 resection [69].
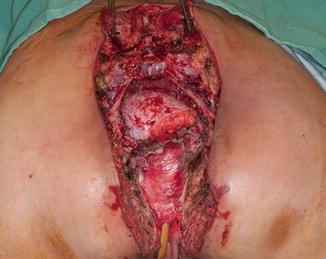

Fig. 51.1
Post-resection photograph showing cut edge of the sacrum, bilateral S3 nerve roots, anterior vaginal wall, and de-epithelialized rectus myocutaneous flap (Copyright held by, and used with permission of, George J. Chang, M.D)
Unfortunately, failure of combined modality therapy portends a poorer prognosis and survival may be suboptimal even with salvage surgery. Several studies have demonstrated 5-year survival rates ranging from 25 to 70 % [70–82]. One of the most important factors affecting recurrence and overall and disease-free survival is involvement of the surgical margins. Other factors include lymph node spread, tumor size, and age [78, 80].
Nodal Recurrence
Regional lymph node recurrence may be identified along the drainage distribution of the anal canal and include mesorectal, inguinal, iliac, or obturator lymph nodes (Fig. 51.2). Mesorectal recurrence often requires proctectomy; whereas inguinal, iliac, or obturator disease may be treated with modified regional lymph node dissection targeting the site of disease without proctectomy. Particularly for intra-pelvic sites of disease, repeat hyper-fractionated chemoradiation therapy may be included prior to salvage surgery as part of the multidisciplinary treatment plan depending on total dose received during initial treatment. Lymph node recurrence, however, occurs more commonly in areas not previously included in the radiation field (e.g., inguinal nodes), and in this case, the treatment of choice is salvage combined modality therapy. Recurrence or persistence following prior definitive radiotherapy may be treated with surgical salvage, including lymph node dissection. Inguinal lymph node dissection carries with it significant morbidity, primarily local wound complications, such as infection, dehiscence, and lymphocele [83, 84]. In cases with prior radiation, coverage with local myocutaneous flaps can help minimize morbidity [85]. Lymphedema is also a potential complication encountered after surgery.
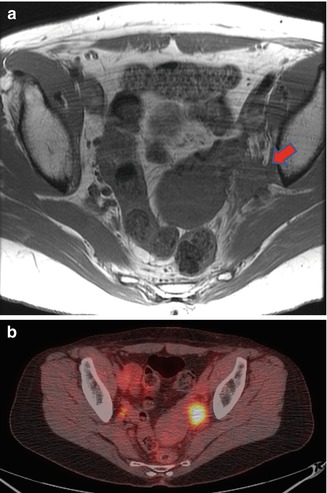

Fig. 51.2
(a) Magnetic resonance imaging shows recurrent carcinoma within left obturator space (red arrow). (b) The same area is shown to be fluoro-deoxyglucose avid on positron emission tomography (Copyright held by, and used with permission of, George J. Chang, M.D)
Distant Metastatic Recurrence
As many as 20 % of patients can have distant metastatic disease at the time of initial presentation or as evidence of recurrence. The most common site of metastasis is the liver, but spread to the lung, peritoneum, and bone also occurs. The mainstay of therapy is systemic chemotherapy. Surgical resection can be performed in select patients, but prognosis overall is poor [86]. Our preference is to begin with systemic chemotherapy and consider salvage surgical resection only in patients who have demonstrated limited disease responsive to systemic therapy.
51.11 Pelvic Reconstruction
Perineal wound dehiscence occurs in as many as 70–80 % of patients. Prior pelvic radiation is a key contributing factor. There are many reconstructive options developed to facilitate perineal wound healing and decrease the incidence of wound complications. The most commonly used are myocutaneous flaps, such as the vertical rectus abdominis myocutaneous (VRAM) flap, rotational anterolateral thigh flaps, and the gracilis myocutaneous flap (Fig. 51.3). VRAM flaps have the major advantage of providing well vascularized soft-tissue volume with excellent blood supply and associated skin coverage for the perineum [87–89]. Similarly, anterolateral thigh flaps have good blood supply but are more limited by length of the vascular pedicle, particularly in shorter patients. While the gracilis flap can provide good coverage for the perineum, it has little bulk to fill the pelvis [90]. Another option is de-epithelialized gluteal advancement, but it similarly has limited space filling of the true pelvis and may require augmentation with a pedicled omental flap. Several comparative studies of flap reconstruction versus primary closure have demonstrated decreased perineal wound complications with flap reconstruction [87–90]. At MD Anderson Cancer Center, our preferred approach includes routine vertical rectus or anterolateral thigh flap reconstruction after salvage APR.