Patients who have a previously negative biopsy in the setting of clinical suspicion of prostate cancer still have a high risk of harboring significant undiagnosed disease. Various markers such as prostate-specific antigen (PSA) velocity, PSA density, PCA3, and newer markers may aid in repeat biopsy selection. Repeating the same biopsy procedure in such patients does not yield high cancer detection rates. More anteriorly directed transrectal or transperineal biopsies are indicated. Multiparametric magnetic resonance imaging can detect abnormal areas, and lesion-targeted biopsies can improve the cancer detection rate.
Key points
- •
Percent free prostate-specific antigen (PSA), PSA velocity, PSA density, and PCA3 can suggest further risk of malignancy in patients with previous negative biopsy.
- •
Repeat biopsy should be directed to areas not previously sampled, such as anterior part, extreme apex and base, and midline.
- •
Changing the route of biopsy to a transperineal approach may improve the detection of anteriorly located cancers.
- •
Multiparametric magnetic resonance imaging (MRI) shows the cancer location with higher sensitivity than transrectal ultrasonography (TRUS) and should be considered before repeat biopsy.
- •
Newer tumor markers, field defect markers and MRI/TRUS fusion technology may improve sensitivity and specificity of detection of prostate cancer.
Introduction
Persistent increase in prostate-specific antigen (PSA) levels in patients with previous negative biopsies creates a clinical dilemma. Increase in PSA levels is nonspecific and can be associated with benign causes, such as benign prostatic hyperplasia, infection, inflammation, infarction, mechanical stimulation, and so forth. Despite its vague implications, urologists are compelled to evaluate increasing PSA levels to avoid missing a diagnosis of prostate cancer. With the recent increase in septicemia cases associated with prostate biopsies, complications can be costly and potentially life threatening. With conventional transrectal ultrasonography (TRUS) technology, sampling errors are inevitable, and a negative biopsy does not rule out malignancy with certainty. The management of the patient with repeatedly negative prostate biopsies and clinical characteristics suggestive of cancer, such as an increased PSA level or abnormal digital rectal examination (DRE), remains a challenging problem for physicians and patients. The current TRUS-guided prostate biopsy technique may be associated with some discomfort and pain. Further, potential complications associated with biopsies are not negligible. Which findings necessitate repeat biopsy and when repeat biopsies should be recommended is difficult to determine. With low certainty of finding a high-risk cancer on repeat biopsy, the benefits of determining a diagnosis must be weighed against the risks of subjecting patients to rebiopsy-related morbidities.
Introduction
Persistent increase in prostate-specific antigen (PSA) levels in patients with previous negative biopsies creates a clinical dilemma. Increase in PSA levels is nonspecific and can be associated with benign causes, such as benign prostatic hyperplasia, infection, inflammation, infarction, mechanical stimulation, and so forth. Despite its vague implications, urologists are compelled to evaluate increasing PSA levels to avoid missing a diagnosis of prostate cancer. With the recent increase in septicemia cases associated with prostate biopsies, complications can be costly and potentially life threatening. With conventional transrectal ultrasonography (TRUS) technology, sampling errors are inevitable, and a negative biopsy does not rule out malignancy with certainty. The management of the patient with repeatedly negative prostate biopsies and clinical characteristics suggestive of cancer, such as an increased PSA level or abnormal digital rectal examination (DRE), remains a challenging problem for physicians and patients. The current TRUS-guided prostate biopsy technique may be associated with some discomfort and pain. Further, potential complications associated with biopsies are not negligible. Which findings necessitate repeat biopsy and when repeat biopsies should be recommended is difficult to determine. With low certainty of finding a high-risk cancer on repeat biopsy, the benefits of determining a diagnosis must be weighed against the risks of subjecting patients to rebiopsy-related morbidities.
Risk of false-negative results with prostate biopsy
Repeat prostate biopsies detect cancer in 16% to 41% of cases in which the initial biopsy was negative. At the University of California at San Francisco (UCSF), Shinohara and colleagues attempted to identify predictors of positive biopsy to avoid unnecessary biopsy procedures in patients at low risk for malignancy by studying 325 men with a history of 2 or more negative biopsies. The mean age of this patient population was 61 years, with a mean serum PSA level of 13.8. The repeat positive biopsy rate in these patients was 38%. The percentage of patients with a positive biopsy decreased as the number of previous negative biopsies increased: 40% in patients with 2 or 3 previous negative biopsies, 36% in patients with 4 or 5 previous negative biopsies, and 17% in patients with 6 previous negative biopsies. Using a Cox proportional hazards model, predictors of positive biopsy were identified, including higher serum PSA level, increased age, hypoechoic lesions on ultrasonography, and smaller prostates. Abnormal pathology (prostatic intraepithelial neoplasia [PIN], atypical small acinar proliferation [ASAP]) on previous biopsy, abnormal DRE, and transition zone volume were not significant predictors of a positive biopsy (Shinohara K, unpublished data, 2004). Clinical data accumulation now identifies more reliable predictors for patients who need a repeat biopsy in this population. Ploussard and colleagues reported the factors associated with repeat biopsy on longitudinal follow-up among patients who had initially negative biopsy. Of 617 men followed for a mean of 19 months, 31% underwent repeat biopsy. The risk factors for repeat biopsy are high PSA levels, high PSA density (PSAD), and younger age. These investigators also reported PSA levels greater than 6 ng/mL, PSAD greater than 0.15, prostate volume less than 50 mL were associated with positive biopsy results.
Predictors for repeat prostate biopsy
High-Grade PIN and ASAP
High-grade PIN (HGPIN) is found on a varying but significant fraction of prostate biopsies (1%–25%), with most modern series having an average of 5%. PIN is characterized by architecturally begin prostate acini, which are lined by cytologically atypical cells. HGPIN may be a precursor lesion to adenocarcinoma. Previously, the discovery of HGPIN on first prostate biopsy prompted repeat prostate biopsy in 3 to 6 months. Published series of those with HGPIN who undergo repeat biopsies show a cancer detection rate of 30% to 50%. However, Lefkowitz and colleagues reported that with an extended biopsy scheme showing HGPIN, repeat biopsy showed cancer in only 2.3% of cases. These investigators recommended that immediate repeat biopsy is not necessary after a 12-core biopsy showing HGPIN. Netto and Epstein reported a higher incidence of cancer diagnosis in patients with initial biopsy showing widespread HGPIN. More recently, Lee and colleagues reported their results of 328 men undergoing repeat prostate biopsy after the initial biopsy showed HGPIN. In their study, these investigators found that a group with multifocal or bilateral HGPIN on initial biopsy had a significantly increased hazard ratio of subsequent prostate cancer compared with the unifocal HGPIN disease group. These investigators found a 3-year cancer detection rate of 29% to 37% in the multifocal HGPIN group.
ASAP, which has also previously been termed atypical adenomatous hyperplasia or atypia, is characterized by the crowding and proliferation of small glands; however, cytologic atypia is minimal. This lesion has been less well characterized than HGPIN, but ASAP alone is identified in 5% of patients undergoing needle biopsy. Iczkowski and colleagues proposed further classification of this lesion into 3 categories (favoring benign, uncertain, and favoring malignant) and suggested correlation of each category with subsequent cancer detection. The association of ASAP with prostate cancer is higher than that of HGPIN. Contemporary biopsy series looking at the influence of ASAP have shown that the probability of detecting adenocarcinoma on repeat biopsy is 40% to 50%. Having both HGPIN and atypia together on the first biopsy may increase the rate of cancer detection on the second biopsy to as high as 75%.
The current indications for repeat biopsy within the first year based on National Comprehensive Cancer Network (NCCN) Guideline Version 2012 include ASAP found on initial biopsy and extensive (multiple biopsy sites, ≥2 cores) HGPIN lesions.
DRE, PSA and PSA Derivatives
Abnormal DRE or abnormal PSA values lack specificity for detecting prostate cancer, especially after the first negative biopsy, with the positive predictive value of PSA detecting clinically significant cancer ranging from 25% to 40%. A PSA level in the range between 4 and 10 most often resulted in a 60% to 70% negative biopsy rate. Prostate cancer is also detected in 17% to 27% of patients with PSA levels from 1 to 4 ng/mL. Furthermore, a repeat saturation biopsy also detected about 18% to 43% of cancers missed after the first biopsy in men with increased PSA levels. Hence, PSA and DRE alone are not ideal tools for reliably excluding cancer. In men with persistently increased PSA levels with 2 or more negative biopsies, clinicians should consider the PSA velocity (PSAV), the adequacy of the initial biopsy (number of cores and location of biopsies), PSAD, family history, age, and African American ethnicity when recommending repeat biopsy. Recent studies have shown that PSAV is a strong predictor of prostate cancer in repeat biopsy after controlling for age, presence of ASAP lesion, PSAD, and percent free PSA at time of repeat biopsy. A mean PSAV of 0.73 ng/mL/y was associated with low-grade cancer on repeat biopsy, whereas a PSAV of 5.73 ng/mL/y was associated with intermediate-grade or high-grade cancer after an initial negative biopsy. Previously, Loeb and colleagues reported that a PSAV greater than 0.4 ng/mL/y is associated with increased risk of Gleason 7 or higher prostate cancer at time of radical prostatectomy. For men with PSA less than 4.0 ng/mL, data suggest that PSAV of 0.35 ng/mL/y or greater is suspicious for the presence of life-threatening cancer, whereas for men with PSA 4 to 10 ng/mL, a PSAV of 0.75 ng/mL/y or greater is suspicious. A recent study from the Cleveland Clinic also supported the significance of PSAV in detection of high-grade cancer among the repeat biopsy population. Hence, PSAV should be considered when selecting high-risk men for repeat biopsy. In addition to suspicious lesions, including ASAP and HGPIN, PSAD and prostate volume have also been shown to be strong predictors of prostate cancer and thus could aid clinicians in optimizing repeat biopsy decisions.
Percent free PSA has been studied extensively in the past. The NCCN guidelines recommend repeat biopsy if percent free PSA is less than 10%. However, free PSA alone still lacks high specificity for prostate cancer. Within free PSA isoforms, [2]proPSA is a main isoform associated with prostate cancer. The prostate health index (PHI) is a test approved by the US Food and Drug Administration (FDA) that calculates the risk of prostate cancer by a formula of [2]proPSA/free PSA×√PSA and has shown better sensitivity and specificity over free PSA or PSA alone. Scattoni and colleagues evaluated the performance of PHI and PCA3 in detection of prostate cancer among 211 patients who underwent biopsy and reported that PHI was more accurate than PCA3 in detecting prostate cancer in the initial biopsy setting as well as repeat biopsy setting.
PCA3
The urine-based PCA3 (Progensa) is available and FDA approved for use in risk stratification for selecting patients for repeat biopsy, with a cutoff value of 25 (a higher score is associated with a higher probability of finding cancer on repeat biopsy). PCA3 is a noncoding messenger RNA (mRNA) that is overexpressed in 95% of prostate cancer. The PCA3 mRNA level is measured using reverse transcription polymerase chain reaction to amplify mRNA in a urine sample after prostate massage. The score is then calculated based on total PSA mRNA concentrations. Multi-institutional validation of the PCA3 test showed that a median score of 20 was associated with negative repeat biopsy and a score of 48 was associated with positive repeat biopsy. Hence, PCA3 seems to be superior to PSA alone when selecting patients for repeat biopsy and may aid urologists and patients in making repeat biopsy decisions. Wu and colleagues reported on 103 patients with previous multiple negative biopsies that PCA3 with a cutoff value of 25 showed sensitivity of 67% and specificity of 64%. In that analysis, PCA3, PSAD, PSA (inverse), DRE, and TRUS were all independently predictive and were included in a multivariable nomogram. The results were significantly better than PSA alone and as sensitive and specific as PSAD. However, Roobol and colleagues reported that if PCA3 was excessively high (>100), the positive predictive value of the test was only 51%. Evaluation of the Genomic Applications in Practice Prevention Working Group reported that the PCA3 test does not have enough clinical evidence to recommend clinical use unless further evidence is acquired to support improved outcomes at this point.
TMPRSS2-ERG Fusion
Still in the clinical trial phase, the TMPRSS2-ERG (transmembrane protease serine 2 implicated in tumor metastasis) fusion urine test is being developed to further refine risk stratification in selecting patients for biopsy. TMPRSS2-ERG fusion is reportedly one of the earliest events in prostate cancer tumorigenesis and is detected in approximately 50% of prostate cancer. When used in combination, the TMPRSS2-ERG fusion and PCA3 urine test can give up to 90% specificity and 80% sensitivity. This could be an attractive tool in the future for selecting men for repeat biopsy. The Mi-Prostate Score is derived from TMPRSS2-ERG fusion combined with PSA and PCA3 results and is commercially available; however, as with PCA3, more clinical data are required before recommending widespread clinical use.
Cancer Field Defect Markers
Benign tissue surrounding cancer areas has been known to have some molecular-level alterations (field defect). By detecting those defects in negative biopsy samples, prostates with probable undiagnosed cancer may be identifiable. DNA hypermethylation in the promoter regions of cancer-associated genes is linked to prostate cancer. Stewart and colleagues studied an epigenetic assay evaluating such DNA hypermethylation in 498 patients who had a previously negative biopsy and underwent repeat biopsies within 30 months. Methylation-specific polymerase chain reaction assay panel was performed on initial negative biopsy samples and correlated with the second biopsy outcomes. These investigators concluded that the negative predictive value of the epigenetic assay was 90%, and unnecessary biopsies can be reliably avoided by using the test (ConfirmMDx, MDx Health, Irvine, CA). Another study using a different field effect marker showed that a 3.4-Kb mitochondrial genome deletion is found in benign tissue surrounding prostate cancer. This field defect can be used as a surrogate for prostate cancer in benign biopsy specimens. If the accumulated data show the field defect markers to be clinically useful, the described tests may reduce unnecessary repeat biopsy rates and be able to target suspicious areas with high predictability.
Advanced imaging
Color Doppler and Power Doppler Imaging
Color Doppler was first described in 1993 as a potential means of differentiating malignant tissue from benign growth. It interprets reflected sound as a measure of blood flow in prostatic vessels. It takes advantage of the hypervascular nature of malignant prostatic tissue to visualize vascular flow. Increased angiogenesis in prostate cancer tissue results in higher microvessel density when compared with that of benign tissue. Initial studies indicated that color Doppler could be used to identify cancers such as isoechoic, hypervascular tumors, which are not visible using conventional gray-scale ultrasonography. However, the data supporting the routine use of color Doppler are not conclusive.
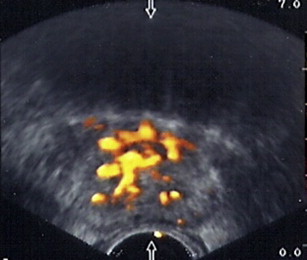
Power Doppler imaging makes use of detecting and amplifying small differences between blood flow in different vessels, allowing imaging of very small tumor vessels ( Fig. 1 ). This modality results in a sensitivity that is 3-fold to 4-fold that of color Doppler alone. Okihara and colleagues reported a sensitivity of 98% and a negative predictive value of 98%, significantly higher than that of gray-scale ultrasonography for visualization of prostate cancer. However, in their study, the positive predictive value was 59%, equivalent to that of gray-scale ultrasonography. In addition, the patient population contained higher volume disease compared with the current PSA-screened population. TRUS studies with microbubble contrast agent enhancement have been reported. Mitterberger and colleagues used contrast-enhanced Doppler imaging in 690 patients and performed both Doppler image-guided biopsies and systemic random biopsies. Targeted biopsy yielded significantly higher positive results and higher Gleason grade disease with fewer biopsy cores compared with random biopsy. Aigner and colleagues used prostate biopsy under contrast-enhanced ultrasonography. Targeted biopsy yielded nearly 50% positive, whereas systematic random biopsy had only 9.3% positive. The data suggest appealing future clinical applications of ultrasound contrast agents; however, there is no FDA-approved ultrasound contrast agent available for prostate imaging in the United States.
Elastography
Elastography measures signal displacement when tissue is slightly compressed during ultrasonographic examination. This modality discriminates hard tissue unlikely to be displaced from soft surrounding tissue. Salomon and colleagues compared elastography imaging with radical prostatectomy specimens in 109 patients. Elastography detected 439 lesions with sensitivity and specificity of 75.4% and 76.6%, respectively. However, when elastography was applied to targeted biopsies, 54% of random biopsies that were found to be positive did not have abnormal findings on elastography. Nelson and colleagues concluded that elastography targeted biopsy could not replace systemic biopsy in the report.
Multiparametric Magnetic Resonance Imaging
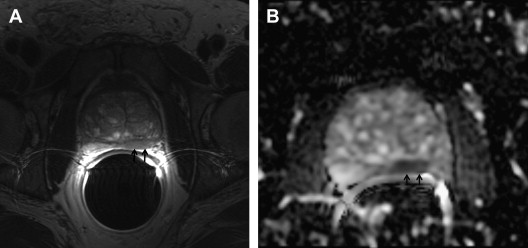
Potential roles for magnetic resonance imaging (MRI) and MRI-guided biopsy (MRI-GB) in the diagnosis, staging, and management of prostate cancer have been evaluated. T2-weighted imaging improved detection of prostate cancer in men with previous negative biopsies, with a detection rate of 55%. More recently, multiparametric MRI (mp-MRI), which combines T2-weighted imaging with diffusion-weighted image (DWI), dynamic contrast-enhanced (DCE) imaging, or proton magnetic resonance spectroscopy (MRS) has shown increased specificity compared with T2-weighted imaging alone. A typical cancer is seen as an area of decreased signal intensity on T2-weighted images, restricted diffusion on DWI, rapid uptake and rapid release of contrast on DCE imaging, and increased choline+creatine/citrate ratio ( Fig. 2 , Table 1 ).
| Characteristics | Cancer Appearance | |
|---|---|---|
| T2-weighted imaging | Every tissue has its own T1 and T2 values, which can be used to generate contrast between tissues | Decreased signal intensity (dark) |
| DWI | Molecular diffusion of water through tissues | Restricted diffusion (bright on longer b value, dark on apparent diffusion coefficient map) |
| DCE imaging | Rapid T1 images are obtained after administration of contrast agent | Early enhancement and early washout of contrast |
| MRS | Proton spectroscopy assesses relative molecular signatures of tissues | Increased choline+creatine/citrate ratios |
Several prospective studies have reported mp-MRI cancer detection rates between 39% and 59% in previously negative biopsy patients and have found that triple combinations of multiparametric imaging modalities detect more cancers than use of only 1 or 2 of the modalities. Again, most studies reported a prevalence of anteriorly located tumors. MRI-GB has relatively high cancer detection rates, detects clinically significant disease, and may require fewer cores.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




