Molecular lesion
Type I (%)
Type II (%)
References
PIK3CA
Mutation
30
20
Amplification
2–14
46
KRAS mutation
11–26
2
AKT mutation
3
Undetected
[85]
PTEN functional loss
50–83
5
FGFR2 mutation
12–16
1
TP53 mutation
20
90
Microsatellite instability
20–45
<5
HER2
Overexpression
3–10
32
Amplification
1
17
[131]
In order to improve outcomes, patient selection according to histologic morphology, even with the known limitation of diagnostic reproducibility [37–39], may be a better approach whilst developing an understanding of the predominant molecular drivers in endometrial malignancy that predict prognostic or therapeutic outcome.
There remains currently a significant unmet need for tolerable, effective therapy to treat patients with advanced endometrial cancer. Due to the relatively poor results with chemotherapy regimens, many classes of novel targeted therapy are being explored in this indication. These include anti-angiogenics, EGFR inhibitors, HER2 inhibitors and most extensively, drugs targeting the PI3K/AKT/mTOR pathway [14, 15, 19, 21, 33, 40, 41].
The PTEN/PI3K/AKT/mTor axis is the most commonly disrupted signaling pathway in endometrial cancer and as a result has received the greatest amount of clinical attention in terms of drug development [33, 42]. Several drugs targeting this signaling pathway are currently being evaluated in the setting of advanced disease and some trials have reported results [19, 41, 43, 44]. The current experience, status, limitations and future directions of novel therapeutics targeting the PTEN/PI3K/AKT/mTor pathway in endometrial cancer will be explored. The importance of continuously evaluating our understanding of endometrial tumour biology and integrating predictive biomarker identification and development with the clinical development of these agents to improve outcomes by directing patient selection will be discussed.
31.2 Strategies for the Medical Management of Advanced Endometrial Cancer
Chemotherapy
There is currently no standard first line chemotherapy in advanced or recurrent disease [1, 8, 10–15, 28, 29, 33]. Although Phase II trials have demonstrated activity with doxorubicin, paclitaxel and platinum compounds, only paclitaxel-containing regimens have consistently shown a response rate (RR) >20 % in advanced disease [1, 45, 46]. Consideration of alternative schedules of chemotherapy may help to improve response rates, with weekly carboplatin-paclitaxel being the most promising with RR up to 71 % in one study and an acceptable toxicity profile [47–50]. Combination chemotherapeutic regimens are generally more active in endometrial cancer than monotherapies, but this is at the expense of greater toxicity. One systematic review of eight randomized controlled trials (RCT) showed that treatment consisting of ‘more’ chemotherapy was associated with longer OS and progression free survival (PFS) [29] although no definitive recommendation could be made for a specific regimen [8, 29]. The GOG177 trial showed that combination of cisplatin/paclitaxel/doxorubicin had significantly greater RR (57 % vs 34 %) and OS (15.3 months vs 12.3 months) compared to doxorubicin/cisplatin but the utility of this regimen is compromised by significant toxicity [51]. Three meta-analyses conclude there is currently no statistically significant evidence to suggest one particular doublet chemotherapy over any other doublet, or a particular single-agent chemotherapy over another [8, 28, 29]. However, data for making comparisons is limited [8, 28, 29]. In view of the substantial side-effect profile of three drug regimens, recurrent endometrial cancer is most commonly treated with a combination of carboplatin/paclitaxel and less commonly, a doxorubicin-containing doublet [40].
Endocrine Therapy
A systematic review of the use of endocrine therapy for the management of endometrial cancer concluded that there is insufficient evidence that hormonal treatment improves the survival of patients with advanced or recurrent disease [9]. However, the authors concede the conclusions are limited by the small patient numbers on RCT which limit the ability to prove a significant benefit [9]. GOG153, a Phase II trial evaluating patients receiving MPA (medroxyprogesterone acetate) alternating with tamoxifen, reported meaningful activity where RR was observed to be 27 % and OS 14 months [52].
Biological Therapy
Preliminary data for several molecular targeted agents in endometrial cancer are emerging [14, 15, 19, 21, 33, 40, 41]. Although a number of established drug targets are expressed in endometrial cancer, therapeutic targeting of several of these in an unselected endometrial cancer population has not yielded much activity. The epidermal growth factor receptor (EGFR) is frequently expressed in normal endometrium as well as endometrial cancer [53] but use of the EGFR inhibitor erlotinib was associated with a RR of only 13 % [54]. Similarly, although HER-2 is overexpressed or amplified in a proportion of endometrial cancers [55], there were no objective responses to trastuzumab in a phase II trial of a selected population of HER-2 positive endometrial cancer patients [56]. Evidence suggests that angiogenesis and vascular endothelial growth factor (VEGF) signaling have a key role in endometrial cancer progression [57]. Two Phase II clinical trials have shown very encouraging activity of bevacizumab monotherapy in advanced or recurrent endometrial cancer [58, 59] making this one of the most promising novel targets for monotherapy in endometrial cancer. Several trials with bevacizumab in endometrial cancer are continuing [40]. A Phase II trial of the antiangiogenic agent sorafenib, an oral, multitargeted tyrosine kinase inhibitor showed disappointing activity in the endometrial population [60]. More recent trials are tending to select an appropriate patient population based on prospective mutational status of a molecular target, e.g. FGFR2 (dovitinib) or PI3K (PF-04691502) when targeting specific pathways (Table 31.2), a practice that is likely to increase.
Table 31.2
Selected current trials of PI3K/AKT/mTOR pathway inhibitors as monotherapy and in combination for endometrial cancer
Drug | Target | Phase | clinicaltrials.gov identifier |
|---|---|---|---|
BKM120 | PI3K | II | NCT01289041 |
XL147 | PI3K | II | NCT01013324 |
PF-05212384 | Dual mTor/PI3K | II | NCT01420081 |
GDC-0980 | Dual mTor/PI3K | II | NCT01455493 |
DS7423 | Dual mTor/PI3K | I | NCT01364844 |
MK2206 | Akt inhibitor | II | NCT01307631 |
Temsirolimus | mTOR | II | NCT01460979 |
Ridaforolimus | mTOR | II | NCT00122343 |
Everolimus | mTOR | II | NCT00087685 |
Temsirolimus/bevacizumab | mTOR/VEGF | II | NCT01010126 |
Temsirolimus/carboplatin/paclitaxel | mTOR/DNA/microtubules | II | NCT00977574 |
Temsirolimus/R04929097 | mTOR/gamma-secretase | I | NCT01198184 |
Temsirolimus/pegylated liposomal doxorubicin | mTOR/DNA | I | NCT00982631 |
Everolimus/letrozole | mTOR/aromatase inhibitor | II | NCT01068249 |
Dovitinib | FGFR2, VEGF | II | NCT01379534 |
Trastuzumab/carboplatin/paclitaxel | HER2/DNA/microtubules | II | NCT01367002 |
31.3 PTEN/PI3K/AKT/mTor Signalling
The PI3K pathway (Fig. 31.1) plays an important role in key cellular functions such as cell growth, proliferation, metabolism and survival [19, 41, 61–63]. Oncogenic dysregulation of the pathway is common in solid tumours due to several different mechanisms of genetic disruption including PIK3CA mutation and PTEN loss [19, 41, 61–63]. Aberrant signalling through the PI3K cascade has also been implicated in chemoresistance in a number of tumour types [19, 41, 61–63]. Within the pathway there are several targets that have been identified for development of novel targeted therapies including mTOR (mammalian target of rapamycin) and PI3K (Fig. 31.1). PTEN (phosphatase and tensin homolog) acts as a suppressor of pathway activation.
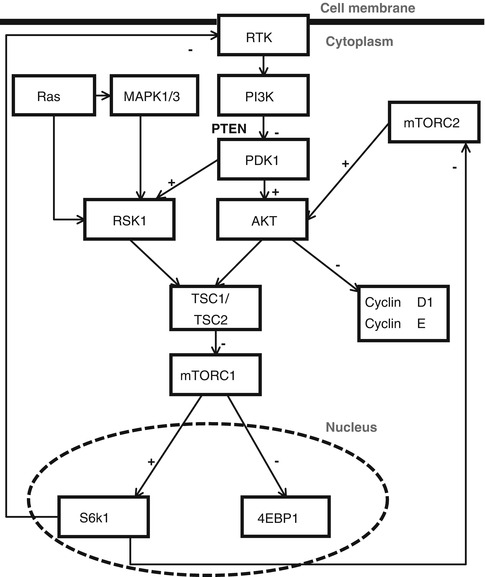

Fig. 31.1
Schematic of the PI3K/mTor pathway. Receptor tyrosine kinases (RTKs) signal through PI3K to activate phosphoinositide-dependent protein kinase-1 (PDK1). PDK1 phosphorylates AKT and ribosomal S6 kinase (RSK1), all of which require a second activating phosphorylation, e.g. by mTORC1, mTORC2, and MAPK3 [133–136]. Thus, PI3K and mTOR pathways act together to promote cell growth, division, and survival [70–72]. TSC1/2 (Tuberous Sclerosis 1/2) functions as a molecular hub, integrating growth factor and energy-sensing pathways to regulate mTOR/Raptor activity [137–141]. The inhibition of mTORC1 does reduce feedback inhibition on upstream regulators and this may be relevant in molecular targeting [132]. High levels of AKT activation leads to survival signals by phosphorylation of several targets including mTORC1 and mTORC2 [132, 142]. mTOR drives cancer growth by activating the lipid and protein biosynthesis needed for robust tumour expansion. This occurs via the resulting hyperactivation of the critical mTORC1 effectors S6K1 (serine 6 kinase 1) and 4EBP1. Oncogenic mTORC1 and mTORC2 activation also drives cell proliferation via increases in cyclin D1 and cyclin E [132, 143, 144]
There are three classes of phosphoinositide 3-kinase (PI3K) each with distinct structure and function. Class IA PI3K heterodimers are the most studied, encoded by the PIK3CA and PIK3R1/PIK3R2 genes for the catalytic (p110) and regulatory (p85) subunits respectively [61, 64, 65]. PTEN is a lipid phosphatase that cleaves phosphoinostides, negatively regulating PI3K-dependent signalling [61, 64, 65]. Phosphorylation of PI3K substrates activates signalling through AKT which initiates a cascade of downstream events [61, 62, 64, 65]. mTOR complex 1 (mTORC1) is one of the main effectors of AKT signaling, mediating lipid and protein synthesis, whilst mTORC2 is part of a positive feedback loop that activates AKT [65–67] which can be problematic in therapeutic targeting of mTORC1 [68, 69].
31.4 The PI3K Pathway and Endometrial Cancer
Alterations in the PTEN/PI3K/AKT/mTor pathway are frequent in Type I and Type II endometrial cancers, often co-exist and are involved in pathogenesis of the disease [20, 33, 42, 73].
One of the most important mechanisms of endometrial carcinogenesis is functional inactivation of PTEN which is often present in precancers suggesting a central role in pathogenesis [73]. PTEN loss is associated with improved prognosis in endometrial malignancy [74, 75] and occurs as a result of various mechanisms including gene mutation, promoter methylation and protein degradation [33, 76, 77]. Although inactivation is rare in normal endometrium, it is present in 20 % of endometrial hyperplasia, 35–50 % of endometrioid endometrial cancer (EEC), and 10 % of non-endometrioid endometrial cancer (NEEC) [20, 25, 26, 33, 63, 76–79].
Activating PIK3CA mutations are estimated to be present in approximately 30 % of EEC and 15 % of NEEC and are frequently coexistent with dysfunctional PTEN [20, 33, 80, 81]. Mutation of the PIK3CA gene is associated with poor survival and an aggressive disease course [42, 82, 83]. The PIK3R1 and PIK3R2 genes encoding for p85α[alpha] and p85β[beta] respectively, regulatory subunits of PI3K, have been found to be amplified and mutated in endometrial cancer, the latter sometimes in the absence of PTEN loss [75, 84]. Novel activating mutations continue to be identified [76].
31.5 Therapeutics Targeting the PI3K/AKT/mTor Pathway in Endometrial Cancer
There are four main categories of novel drug that target different proteins in the PTEN/PI3K/AKT/mTor pathway (Fig. 31.2) with varying amounts of clinical experience for each: mTor inhibitors, PI3K inhibitors, dual PI3K/mTor inhibitors, AKT inhibitors. Combination therapies with these agents are also being clinically explored.
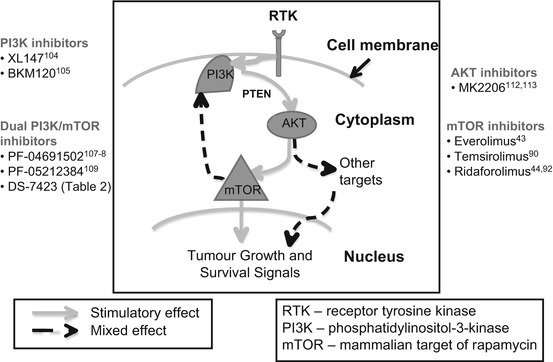

Fig. 31.2
PI3K/mTOR therapeutic agents in endometrial cancer
mTOR Inhibitors
Of all drugs targeting the PI3K pathway, the first generation mTor inhibitors (rapamycin analogues) have been the most extensively evaluated in endometrial cancer. Second generation compounds targeting mTor catalytic function through the mTor kinase domain, inhibit mTorc1/mTorc2 simultaneously and demonstrate greater potency [87–89]. Dual targeting of mTorc1 and mTorc2 circumvents the unwanted positive feedback loop where uninhibited mTorc2 stimulates AKT activation as can occur with the rapalogs [68, 69].
Temsirolimus [90], ridaforolimus [44, 91, 92] and everolimus [43] have been evaluated as monotherapy in unselected patients with inoperable advanced or metastatic endometrial cancer. Objective response rates were 14 % (temsirolimus [90]) and 4.4–7.7 % (ridaforolimus [91, 92]) with no objective response obtained with everolimus [43]. Many patients within these Phase II studies, however, benefited from long term disease stabilisation and those with no prior therapy for advanced disease benefited more [43, 91, 92]. A randomized Phase II ridaforolimus trial demonstrated a statistically significant benefit in progression free survival for ridaforolimus over more standard treatment [44]. Common adverse events were fatigue, rash, mucositis, hyperglycaemia (defined as on-target toxicity) and pneumonitis with hypertriglyceridaemia being less common [43, 44, 90–92]. Neither PTEN nor PIK3CA status correlated with response to temsirolimus [90] and analysis is ongoing for ridaforolimus [92]. Studies examining the relationship of PTEN and PIK3CA mutation as predictors of response to everolimus also found no correlation with outcome [93, 94].
The mechanism of the greater efficacy and potency of second generation mTOR catalytic site inhibitors may be due to their ability to inhibit the rapamycin-resistant mTorc1 functions rather than the additional inhibition of mTorc2 [95, 96]. It was established, however, that inhibition of mTorc2 prevented phosphorylation of Akt [96]. However, these agents had minimal effects on the phosphorylation state of several Akt substrates despite effectively inhibiting Akt S473 phosphorylation, suggesting they may not disable all components of Akt signalling [96, 97]. Other pathways of activation may remain unblocked allowing feedback activation still to occur which may result in hyperactivation of Akt-independent effectors of PI3K signalling [96–98]. mTor kinase inhibitors in early phase trial for solid tumours that include endometrial cancer are AZD2014, OSI-027 and INK128 (clinicaltrials.gov).
PI3K Inhibitors
PI3K inhibitors can be divided into isoform-specific inhibitors or pan-PI3K inhibitors which target all four Class I PI3Ks. Isoform-specific inhibitors are potentially more tumour-specific and have a better side effect profile due to their specificity. For example, p110α[alpha]-specific inhibitors may effectively shut off PI3K–Akt signalling in cancers with PIK3CA mutations [98]. A p110β[beta]-specific inhibitor has shown promise in preventing PI3K signalling in PTEN-deficient cancers [99–102]. However, even in cancers that seem to be specifically reliant on either p110α[alpha] or p110β[beta], there is the concern that other non-targeted p110 isoforms might eventually compensate for decreased activity of the targeted isoform [98]. Given the prevalence of PTEN loss and PIK3CA mutation in endometrial cancers, the isoform-specific inhibitors, e.g. INK1117, NVP-BYL719 and GSK2636771 may be beneficial in this patient population. However, this depends on these genetic mutations predicting for response which has not been the case for mTor inhibition [90, 93, 94].
Phase 2 clinical trials are ongoing with the pan-PI3K inhibitors NVP-BKM120 and XL147 in endometrial cancer (clinicaltrials.gov). The side effect profile of these agents has been tolerable in Phase I studies mainly with nausea, vomiting and g-i upset and manageable hyperglycaemia [103, 104]. The results from the endometrial cancer trials are awaited with interest. Consistent with its mechanism of action, NVP-BKM120 decreases the cellular levels of p-Akt and preferentially inhibits tumour cells bearing PIK3CA mutations, in contrast to either KRAS or PTEN mutant models [105]. NVP-BKM120 behaves synergistically when combined with either targeted agents such as MEK or HER2 inhibitors or with cytotoxic agents such as docetaxel or temozolomide [105]. These findings may impact future development of this class of drugs.
Dual PI3K/mTOR Inhibitors
Most of the dual PI3K-mTOR inhibitors target the Class I PI3K isoforms (p110α[alpha], p110β[beta] and p110δ[delta] predominantly) as well as mTORC1 and mTORC2 in order to maximally inhibit PI3K pathway signaling. This approach may also minimize the feedback activation of PI3K signaling observed with mTorc1 inhibitors and generate greater therapeutic benefit [68, 106]. This class of inhibitors might also be effective in cancers with Akt mutations or amplifications [98]. The potential advantages of these compounds over other agents targeting the PI3K cascade are being established as the drugs progress through clinical development.
PF-04691502 and PF-05212384 are dual PI3K/mTor inhibitors being investigated in a second line Phase 2 trial in endometrial cancer. In PIK3CA-mutant and PTEN-deleted cancer cell lines, PF-04691502 reduced phosphorylation of AKT inhibited cell proliferation via mTORC1 activity inhibition [107]. In the first in human, Phase I studies both PF-04691502 and PF-05212384 had been well tolerated with the most common treatment-related adverse events being fatigue, nausea, vomiting, decreased appetite, rash, and hyperglycaemia, similar to pan-PI3K inhibitors [108, 109]. GDC-0980 is another dual PI3K/mTor inhibitor [110] found to be tolerable in Phase I [111], also being evaluated in endometrial cancer with results awaited (Table 31.2).
Akt Inhibitors
Although Akt mutation is uncommon in endometrial cancer, this target is being pursued in this indication with MK2206 (Table 31.2). Cancers with AKT1 mutations and AKT1 and AKT2 amplifications might be expected to be more sensitive to Akt inhibitors [98]. MK2206 is a potent inhibitor of Akt 1, 2 and 3, with broad preclinical antitumor activity and tolerability as well as clinical activity in Phase I trial [112, 113]. Common drug-related toxicities include hyperglycemia, skin rash, nausea, fatigue, and diarrhoea [112].
Combination Trials
In an attempt to reduce resistance and enhance efficacy of the various drugs targeting the PI3K pathway, trials investigating combinations with mechanistically diverse agents are underway (clinicaltrials.gov, Table 31.2). The main limitation for this approach is increased toxicity. The role of overactive PI3K signaling in chemoresistance supports the combination of PI3K pathway targeting agents with chemotherapeutics (temsirolimus, carboplatin, paclitaxel) as well as endocrine therapy (letrozole and everolimus) [19]. In addition, combinations of mechanistically complementary agents both with activity in endometrial cancer for example bevacizumab and temsirolimus, are being evaluated.
Limitations of PI3K/mTOR Pathway Inhibitors in Endometrial Cancer
Despite a high incidence of molecular disruption in the PTEN/PI3K/AKT/mTor axis in endometrial cancers, only a small percentage of patients have responded to this class of drugs. One possible reason for this is the lack of patient selection and a tendency towards including patients with mutations in this pathway in clinical trials of PI3K/AKT/mTOR inhibitors has gained some momentum [115]. However, even though these are targeted agents, it is not clear which patients are most likely to benefit as the presence of PIK3CA mutation and PTEN loss have not so far been predictive of clinical benefit [90, 93, 94]. One recent report suggests a subtype of PIK3CA mutation, H1047R, may be predictive of response to drugs targeting this pathway but warrants further investigation [116]. This is likely to be a reflection of the complexity of the signalling networks and the multiplicity of feedback loops within and around the pathway [98]. Possibly, these drugs should be investigated in the first line/adjuvant setting, before resistance is established.
Further understanding of the molecular events and drivers in endometrial cancer through initiatives such as The Cancer Genome Project (TCGA) will help to inform biomarker development in order that patients can be appropriately selected for treatment [17, 18]. Validating assays for biomarker assessment and finding cost-effective measures to roll-out testing of samples in a directed way is becoming more important.
The specific toxicity of these drugs has limitations within this patient population. Whilst on the whole they are fairly well-tolerated and orally administered, the potential for hyperglycaemia will be problematic for a proportion of endometrial cancer patients due to pre-existing insulin resistance associated with obesity. Optimal absorption of oral preparations may be impeded by intra-abdominal disease. Other considerations are general ill-health of patients presenting with advanced disease due to other co-morbidities [32]. If used in combination regimens with other drugs toxicity may be enhanced.
31.6 Future Directions
The future of novel agents targeting this molecular pathway in endometrial cancer will depend on the ability to define a sensitive patient population for monotherapy and finding effective, tolerable combination strategies.
Studies have shown that inhibition of mTORC1 also leads to activation of the ERK signalling pathway [114], raising concerns that crosstalk could mitigate the effectiveness of PI3K pathway inhibitors. An increasing amount of preclinical data suggest that activating KRAS mutations may predict resistance [94, 114]. In the latter case, combined inhibition of the RAS/RAF/MEK and PI3K/AKT/mTOR pathways has been suggested as a therapeutic strategy. In addition, the PI3K/AKT/mTOR pathway has been implicated in conferring resistance to conventional therapies, and so PI3K/AKT/mTOR pathway inhibitors in combination with hormonal and/or cytotoxic agents are being evaluated.
Frequent mutations in fibroblast growth factor receptor 2 (FGFR2) in EEC (12 %) also point to the importance of receptor tyrosine kinase (RTK) signalling in the aetiology of this disease [117]. In vitro studies have shown that endometrial cancer cell lines with activating FGFR2 mutations are selectively sensitive to a pan-FGFR inhibitor, PD173074. Several agents with activity against FGFRs are currently in clinical trials [118, 119]. These are also potential therapeutics to consider in combination with PI3K pathway inhibitors although combination toxicity may be problematic.
Angiogenesis is a key component of tumour growth, and metastasis and angiogenic growth factors, such as vascular endothelial growth factor (VEGF), are highly expressed in endometrial carcinomas [57, 120]. VEGF expression has been correlated with poor prognostic factors such as lymphovascular space invasion (LVSI), nodal metastasis and poor survival [120, 121]. Anti-angiogenic agents such as bevacizumab show clinical promise [58] and are being combined with mTor inhibitors in endometrial cancer (Table 31.2).
The first line treatment of disease with biologics is a development direction that may be warranted. In response to evidence supporting combination regimens with cytotoxic and targeted agents, many trials are underway to demonstrate superior efficacy in the first line. One example is the GOG phase II trial, GOG 86P that compares the current standards (carboplatin and a taxol plus bevacizumab or temsirolimus) with carboplatin, bevacizumab and ixebepilone in the first-line treatment of advanced endometrial cancer [14].
31.7 Summary
Drugs targeting the PI3K axis are showing promise in endometrial cancer but are not without toxicity. Understanding how to molecularly select patients for these treatments is likely to improve their clinical benefit. Combination regimens may also broaden their activity for the treatment of endometrial cancer.









