Male Sexual Dysfunction: Introduction
It is estimated that more than half of men aged 40–70 years in the United States are unable to attain or maintain a penile erection sufficient for satisfactory sexual performance. Advances in pharmacologic therapy for erectile dysfunction (ED), coupled with a better understanding of male sexual dysfunction, have resulted in greater numbers of patients seeking care for sexual concerns. Oral phosphodiesterase type-5 inhibitors (PDE-5Is) have emerged as the preferred first-line treatment of ED worldwide due to their efficacy, ease of use, and patient safety. Erectile function can now be evaluated by the response to these agents at home or intracavernous injection (ICI) of vasoactive agents in the office, and improved diagnostic tests can differentiate among types of impotence. Patient satisfaction with penile prostheses is high, as the latest generation of devices is more sophisticated and durable than ever. Current treatments continue to evolve and new therapies such as stem cells and gene therapies may represent the next generation of more physiologic and disease-specific solutions to various types of ED (Bahk et al, 2010; Lin et al, 2009; Melman et al, 2007).
Physiology of Penile Erection
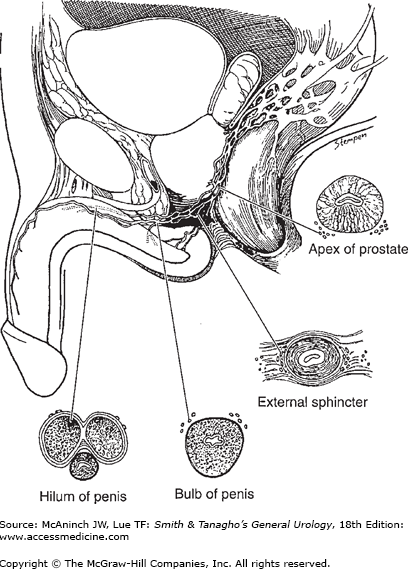
The autonomic spinal erection center is located in the intermediolateral nuclei of the spinal cord at levels S2–S4 and T12–L2. Nerve fibers from the thoracolumbar (sympathetic) and sacral (parasympathetic) spinal segments join to form inferior hypogastric and pelvic plexuses, which send branches to the pelvic organs. The fibers innervating the penis (cavernous nerves) travel along the posterolateral aspect of the seminal vesicles and prostate and then accompany the membranous urethra through the genitourinary diaphragm (Figure 39–1). Some of these fibers enter the corpora cavernosa and corpus spongiosum with the cavernous and bulbourethral arteries. Others travel distally with the dorsal nerve and enter the corpus cavernosum and corpus spongiosum in various locations to supply the mid- and distal portions of the penis. The terminal branches of the cavernous nerves innervate the helicine arteries and trabecular smooth muscle, and are responsible for vascular events during tumescence and detumescence.
The center for somatic motor nerves is located at the ventral horn of the S2–S4 segments (Onuf’s nucleus). The motor fibers join the pudendal nerve to innervate the bulbocavernosus and ischiocavernosus muscles. The somatic sensory nerves originate at receptors in the penis to transmit pain, temperature, touch, and vibratory sensations. The brain has a modulatory effect on the spinal pathways of erection, specifically the medial preoptic area and paraventricular nucleus of the hypothalamus, periaqueductal gray of the midbrain, and the nucleus paragigantocellularis of the medulla. Positron emission tomography and functional magnetic resonance imaging have allowed for greater understanding of brain activation during sexual arousal and orgasm by measuring regional cerebral blood flow or activity. These powerful tools, used in the study of higher brain function and central activation of sexual arousal, may better define pathophysiology associated with varied conditions including psychogenic ED, premature ejaculation, and orgasmic dysfunction (Georgiadis and Holstege, 2005).
Three types of erections are noted in humans: genital stimulated (contact or reflexogenic), central stimulated (noncontact or psychogenic), and central originated (nocturnal). Genital-stimulated erection is induced by tactile stimulation of the genital area. This kind of erection can be preserved in upper spinal cord lesions, although erections are usually short in duration and poorly controlled by the individual. Central-stimulated erection is more complex, resulting from memory, fantasy, visual, or auditory stimuli. Centrally originated erections can occur spontaneously without stimulation or during sleep; most sleep erections occur during rapid eye movement (REM) sleep. During REM sleep, the cholinergic neurons in the lateral pontine tegmentum are activated while the adrenergic neurons in the locus coeruleus and the serotonergic neurons in the midbrain raphe are silent. This differential activation may be responsible for the nocturnal erections during REM sleep. Of note, the number and duration of erections for men with hypogonadism or receiving antiandrogen therapy is markedly reduced (Granata et al, 1997).
The tunica of the corpora cavernosa is a bilayered structure with multiple sublayers. The inner circular bundles support and contain the cavernous tissue. From this inner layer, intracavernosal pillars that act as struts radiate to augment the septum; both structures provide structural support to the corpus cavernosum. The outer-layer bundles are oriented longitudinally and extend from the glans penis to the proximal crura. These fibers insert into the inferior pubic ramus but are absent between the 5- and 7-o’clock positions. In contrast, the corpus spongiosum lacks an outer layer or intracorporeal struts, ensuring a lower pressure structure during erection. The tunica is composed of elastic fibers forming a network on which the collagen fibers rest. Emissary veins run between the inner and outer layers for a short distance, often piercing the outer bundles obliquely. Branches of the dorsal artery take a more direct perpendicular route and are surrounded by a periarterial fibrous sheath (Hsu et al, 2004).
The paired internal pudendal artery is the major carrier of the blood supply to the penis, dividing into three branches: the bulbourethral artery, dorsal artery, and the cavernous artery (deep artery). The cavernous artery supplies the corpora cavernosa; the dorsal artery, the skin, subcutaneous tissue, and the glans penis; and the bulbourethral artery, the corpus spongiosum. In some cases, accessory pudendal arteries from external iliac or obturator arteries may supply a major portion of the penis, with collaterals among the three branches often observed. The venous drainage of the glans is mainly through the deep dorsal vein. The corpus spongiosum is drained via the circumflex, urethral, and bulbar veins, but the drainage of the corpora cavernosa is more complex: the mid- and distal shaft are drained by the deep dorsal and periarterial veins to the preprostatic plexus while the proximal portion is drained by the cavernous and crural veins to the preprostatic plexus and internal pudendal vein. The drainage of all three corpora originates in the subtunical venules, which unite to form emissary veins. The glans penis possesses numerous large and small veins that communicate freely with the dorsal veins. The penile skin and subcutaneous tissue are drained by superficial dorsal veins, which then empty into the saphenous veins.
Activation of the autonomic nerves produces a full erection secondary to filling and trapping of blood in the cavernous bodies. After full erection is achieved, contraction of the ischiocavernosus muscle (from activation of the somatic nerves) compresses the proximal corpora and raises the intracorporal pressure well above the systolic blood pressure, resulting in rigid erection (Table 39–1). This rigid phase occurs naturally during masturbation or sexual intercourse but can also occur from slight bending of the penis, without muscular action. The erection process can be divided into phases as shown in Table 39–1 and Figure 39–2. The hemodynamics of the penile glans is somewhat different. Arterial flow increases in a manner similar to that in the shaft. Because it lacks the tunica albuginea, however, the glans functions as an arteriovenous fistula during the full erection phase. Nevertheless, during rigid erection, most of the venous channels are temporarily compressed, and further engorgement of the glans can be observed (Lue, 2000).
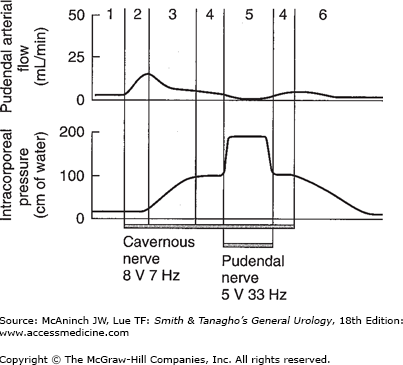
Figure 39–2.
Phases of penile erection (induced in monkeys via neurostimulation). Numbers correspond to phases outlined in Table 39–1. (Lower tracing = intracavernous pressure; upper tracing = flow within the internal pudendal artery.)
Flaccid phase (1) |
Minimal arterial and venous flow; blood gas values equal those of venous blood. |
Latent (filling) phase (2) |
Increased flow in the internal pudendal artery during both systolic and diastolic phases. Decreased pressure in the internal pudendal artery; unchanged intracavernous pressure. Some elongation of the penis. |
Tumescent phase (3) |
Rising intracavernous pressure until full erection is achieved. Penis shows more expansion and elongation with pulsation. The arterial flow decreases as the pressure rises. When intracavernous pressure rises above diastolic pressure, flow occurs only in the systolic phases. |
Full erection phase (4) |
Intracavernous pressure can rise to as much as 90–100% of the systolic pressure. Arterial flow is less than that in the initial filling phase but is still higher than in the flaccid phase. Although the venous channels are mostly compressed except the proximal ones, the total venous flow is slightly higher than venous flow in the flaccid phase. Blood gas values approach those of arterial blood. |
Rigid erection phase (5) |
As a result of contraction of the ischiocavernous muscle, the intracavernous pressure rises well above the systolic pressure, resulting in rigid erection. During this phase, no blood enters the corpus cavernosum. The duration of this phase is short and does not cause ischemia or tissue damage. |
Detumescent phase (6) |
After ejaculation or cessation of erotic stimuli, sympathetic tonic discharge resumes, resulting in contraction of the smooth muscles around the sinusoids and arterioles. This diminishes the arterial flow to flaccid level, reopens the venous channels, and expels blood from the sinusoidal spaces. The penis returns to its flaccid length and girth. |
The penile erectile tissue, specifically cavernous, arteriolar, and arterial wall smooth musculature, is key to the erectile process. In the flaccid state, these smooth muscles are tonically contracted due to intrinsic smooth-muscle tone and possibly tonic adrenergic discharge, allowing only a small amount of arterial flow for nutritional purposes. The blood partial pressure of oxygen (PO2) is about 35 mm Hg. When smooth muscles relax due to the release of neurotransmitters, resistance to incoming flow drops to a minimum. Arterial and arteriolar vasodilatation occurs, and sinusoids expand to receive a large increase of flow. Trapping of blood causes the penis to lengthen and widen rapidly until the capacity of the tunica albuginea is reached. Expansion of the sinusoidal walls against one another and the tunica albuginea results in compression of the subtunical venous plexus. As well, uneven stretching of the layers of the tunica albuginea compresses the emissary veins and effectively reduces the venous flow to a minimum (Lue, 2000; Figures 39–3A and B). Intracavernous pressure (ICP) and PO2 increase to about 100 and 90 mm Hg, respectively, raising the penis from a dependent position to the erect state; further pressure increases (to several hundred millimeters of mercury) due to contraction of the ischiocavernosus muscles result in the rigid-erection phase (Dean and Lue, 2005; Gratzke et al, 2010).
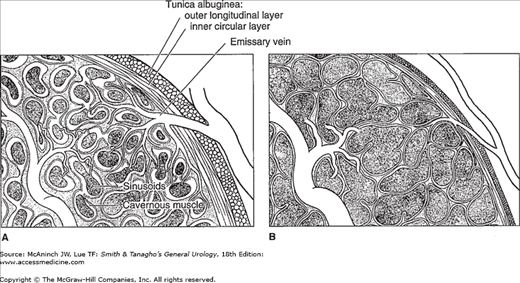
Figure 39–3.
The mechanism of penile erection. In the flaccid state (A), the arteries, arterioles, and sinusoids are contracted. The intersinusoidal and subtunical venular plexuses are wide open, with free flow to the emissary veins. In the erect state (B), the muscles of the sinusoidal wall and the arterioles relax, allowing maximal flow to the compliant sinusoidal spaces. Most of the venules are compressed between the expanding sinusoids. Even the larger intermediary venules are sandwiched and flattened by distended sinusoids and the noncompliant tunica albuginea. This effectively reduces the venous capacity to a minimum.
Androgens are essential for male sexual maturity. Testosterone (T) regulates gonadotropin secretion and muscle development; dihydrotestosterone mediates male sexual maturation, including hair growth, acne, male pattern baldness, and spermatogenesis. In adults, androgen deficiency results in decreased libido (sexual interest) and impaired seminal emission. Aging is associated with a progressive decline of testosterone, dehydroepiandrosterone, thyroxine, melatonin, and growth hormone and increased levels of sex hormone-binding globulin, pituitary gonadotropins, and prolactin (Morales, 2005). In a longitudinal study of middle-aged men for 7–10 years, total T levels declined at 0.8%/year of age, whereas both free and albumin-bound T declined at about 2%/year. Sex hormone-binding globulin increased at 1.6%/year (Feldman et al, 2002). Testosterone levels do not correspond to severity of ED; however, lower levels are observed in men with reduced libido. Although frequency, magnitude, and latency of nocturnal penile erections are reduced with decreased T, erectile response to visual sexual stimulation is preserved in men with hypogonadism, suggesting that androgen is not essential for erection. Due to the inhibitory action of prolactin on central dopaminergic activity and resultant decreases in gonadotropin-releasing hormone secretion, hyperprolactinemia of any cause results in both reproductive and sexual dysfunction (Corona et al, 2004).
Neural control of penile erection involves adrenergic, cholinergic, and nonadrenergic–noncholinergic (NANC) neuroeffector systems. Adrenergic nerves mediate intracavernous smooth-muscle contraction, maintaining the penis in a nonerect state. Currently, it is suggested that sympathetic contraction is mediated by activation of postsynaptic alpha-1a- and alpha-1d-adrenergic receptors and modulated by presynaptic alpha-2-adrenergic receptors (Giuliano et al, 2004). Cholinergic nerves may contribute to smooth-muscle relaxation and penile erection through inhibition of adrenergic nerves via inhibitory interneurons and the release of nitric oxide (NO) from the endothelium by acetylcholine (Saenz de Tejada et al, 2004).
The principal neurotransmitter for penile erection is NO from parasympathetic, NANC nerve terminals. Once blood rushes into the sinusoids, shear stress can also release NO from endothelium to augment smooth-muscle relaxation and erection. In addition, oxygen tension and substances secreted by endothelial cells lining the sinusoidal spaces, prostaglandins, endothelins, and angiotensin may also be involved in penile erection and detumescence (Musicki and Burnett, 2006). The agents capable of inducing erection and causing detumescence are summarized in Table 39–2. Although mechanisms of action vary, erection-inducing substances cause smooth muscle to relax and detumescing agents cause them to contract.
Inducers | Inhibitors |
|---|---|
Papaverine | Phenylephrine |
Phentolamine | Epinephrine |
Phenoxybenzamine | Norepinephrine |
Thymoxamine | Metaraminol |
Alprostadil (prostaglandin E1) | Ephedrine |
Vasoactive intestinal polypeptide (VIP) | Prostaglandin I 2 |
Calcitonin gene-related peptide (CGRP) | Prostaglandin F 2α |
Nitric oxide donor | Thromboxane A 2 (TXA2) |
Guanylate cyclase activator | Endothelin |
Dopamine receptor agonist | Angiotensin II |
Phosphodiesterase inhibitors | |
Rho-kinase inhibitors | |
Melanocortin receptors agonist |
Smooth-muscle contraction is regulated by Ca2+. As cytosolic free Ca2+ increases from resting levels of 120–270 to 500–700 nM, calmodulin-4 Ca2+ complex binds to myosin light-chain kinase. The activated kinase then phosphorylates the light chain and initiates a contraction cycle (Gratzke et al, 2010). Once cytosolic Ca2+ returns to basal levels, calcium-sensitizing pathways take over. Activation of excitatory receptors coupled to G proteins causes contraction by increasing calcium sensitivity without changes in cytosolic Ca2+ levels. This pathway involves RhoA, a small monomeric G protein that activates Rho-kinase. Activated Rho-kinase phosphorylates, and thereby inhibits, the regulatory subunit of smooth-muscle myosin phosphatase, preventing dephosphorylation of myofilaments and maintaining contractile tone (Jin and Burnett, 2006). The emerging consensus is that phasic contraction of penile smooth muscle is regulated by increased cytosolic Ca2+ and tonic contraction is governed by calcium-sensitizing pathways.
During sexual stimulation, NO released from nerve endings and endothelium diffuses into the trabecular and arterial smooth-muscle cells to activate guanylyl cyclase, catalyzing the formation of second messenger cyclic guanosine monophosphate (cGMP). cGMP in turn activates protein kinase G, phosphorylating potassium and calcium channels; the end result is hyperpolarization, reduced intracytosolic calcium, and dissociation of the myosin head from actin as smooth muscle relaxes. Cyclic adenosine monophosphate (cAMP) is another second messenger involved in smooth-muscle relaxation and is activated by cAMP-signaling molecules including adenosine, calcitonin gene-related peptides, and prostaglandins (Lin et al, 2005).
Both of these second messengers activate cAMP- and cGMP-dependent protein kinases, resulting in a drop of cytosolic free calcium and smooth-muscle relaxation via the (1) opening of the potassium channels and hyperpolarization, (2) sequestration of intracellular calcium by the endoplasmic reticulum, and (3) blockage of calcium influx through inhibition of voltage-dependent calcium channels (Dean and Lue, 2005). On the other hand, norepinephrine, phenylephrine, and endothelin appear to activate phospholipase C, leading to the formation of inositol triphosphate and diacylglycerol. The net result is increased cytoplasmic calcium and subsequent smooth-muscle contraction. Detumescence occurs following degradation of cGMP and cAMP to GMP and AMP, respectively, by specific phosphodiesterases. Eleven classes of phosphodiesterases have been identified. The penis is rich in PDE-5 (GMP specific), and therefore, the selective PDE-5Is (sildenafil, vardenafil, and tadalafil) are able to improve penile erections in patients with ED.
Gap junctions are aqueous intercellular channels that have been demonstrated to interconnect the cytoplasm of adjacent cells in many tissues. In the penis, smooth-muscle cells are sparsely innervated by the terminal branches of the cavernous nerves. Therefore, gap junctions play a vital role in the intercellular communication within the corpus cavernosum, enabling the penis to function as a unit (Melman et al, 2007).
Male Sexual Dysfunction
Male sexual dysfunction, denoting the inability to achieve a satisfactory sexual relationship, may involve inadequacy of erection or problems with emission, ejaculation, or orgasm.
Premature (rapid) ejaculation refers to persistent or recurrent occurrence of ejaculation with minimal sexual stimulation before, on, or shortly after penetration and before the person wishes it.
Retrograde ejaculation denotes backflow of semen into the bladder during ejaculation owing to an incompetent bladder neck mechanism.
Epidemiology
In the Massachusetts Male Aging Study, a community-based survey of men between 40 and 70 years of age, 52% of respondents reported some degree of ED: 17% mild, 25% moderate, and 10% complete. Although the prevalence of mild ED remained constant (17%) between the age of 40 and 70, there was a doubling in the number of men reporting moderate ED (from 17% to 34%) and a tripling in the number of men reporting complete ED (from 5% to 15%) (Feldman et al, 1994). More than 70% of men older than 65 years report that they are sexually active; however, 40% are dissatisfied with their sexual function (Braun et al, 2000). Among the major predictors of ED are hypertension, hyperlipidemia, diabetes mellitus, and heart disease. Risk of ED appears to increase with smoking, and may occur in a dose-dependent manner (Polsky et al, 2005). There is a higher prevalence of ED in men who have undergone radiation or surgery for prostate cancer or other pelvic malignancies. The psychological correlates of ED include decreased self-esteem, depression, anxiety, anger, and relationship dissatisfaction (Althof et al, 2006). Other male sexual dysfunctions have also been found to be highly prevalent: premature ejaculation and decreased libido (lack of sexual interest) are common patients’ concerns.
The classification system of ED most commonly used encompasses organic, psychogenic, and mixed etiologies of ED and is endorsed by the International Society of Impotence Research (Table 39–3). In the 1950s, 90% of cases of ED were believed to be psychogenic. Most authors now believe that mixed organic and psychogenic ED is the most common.
|
Many psychologic conditions (performance anxiety, strained relationship, lack of sexual arousal, depression, and schizophrenia) can either cause or aggravate ED. Sexual behavior and penile erection are controlled by the hypothalamus, cerebral cortex, and limbic systems. Given the number and complexity of known and as yet unidentified factors involved, it is not surprising that the pathogenesis of psychogenic ED is still speculative. Possible mechanisms proposed include an imbalance of central neurotransmitters, overinhibition of spinal erection center by the brain, inadequate NO release, and sympathetic overactivity (Bodie et al, 2003).
It has been estimated that up to 20% of all ED is neurogenic in origin, resulting from peripheral (cavernous and pudendal nerve) or central pathologies. In men with spinal cord injury, the degree of erectile function depends on the nature, location, and the extent of the lesion. Brain lesions associated with ED include dementias, Parkinson’s disease, stroke, tumors, trauma, and Shy-Drager syndrome (Papatsoris et al, 2006). Peripheral neuropathy due to diabetes mellitus, chronic alcohol abuse, or vitamin deficiency may affect the nerve endings and result in a deficiency of neurotransmitters. Direct injury to the cavernous or pudendal nerves from trauma, radical pelvic surgeries for malignancy, or pelvic irradiation can also cause ED. It is important to note that even with nerve-sparing approaches to prostatic and rectal surgery, erectile recovery can take up to 24 months or longer.
Historically, hypogonadism as a cause of ED was thought to be rare, but recent data support a significant increase of hypogonadism with age. Hypogonadism due to hypothalamic or pituitary tumors, estrogen or antiandrogen therapy, or orchiectomy can suppress sexual interest and nocturnal erections. As mentioned earlier, erections are usually preserved to some extent. Hyperprolactinemia, Cushing’s syndrome, and Addison’s disease can cause decreased libido and ED. Hyperthyroidism is commonly associated with decreased libido, likely due to elevated estrogen levels, while hypothyroidism can contribute to ED through diminished testosterone secretion and elevated prolactin levels (Veronelli et al, 2006).
Although arteriogenic ED may be congenital or posttraumatic, more often it is part of a generalized systemic arterial disease. Traumatic arterial occlusive or atherosclerotic disease of the hypogastric (iliac)–pudendal–cavernous arterial tree can decrease flow to the sinusoidal spaces and perfusion pressure, thus decreasing the rigidity or prolonging time to maximal erection. Some patients with severe arterial disease may retain erectile function as long as arterial flow exceeds venous flow; conversely, some patients with minimal arterial disease may develop partial or complete ED because of large venous outflow, cavernous smooth-muscle dysfunction, or inadequate neurotransmitter release (Dean and Lue, 2005).
High prevalence of ED has been reported in men with coronary, cerebral, and peripheral vascular diseases (Chai et al, 2009; Kloner et al, 2003). Among men with coronary arterial disease, the prevalence of ED increases as the severity of coronary arterial lesions increase. In patients with chronic coronary disease who also had ED, onset of sexual dysfunction occurred before coronary artery disease (CAD) onset in 93%, with a mean time interval of 24 [12–36] months (Montorsi et al, 2006). When ED occurs in a younger man, it is associated with a marked increase in the risk of future cardiac events, whereas in older men, ED appears to be of little prognostic importance (Inman et al, 2009).
Common risk factors associated with arterial insufficiency include hypertension, hyperlipidemia, diabetes mellitus, and metabolic syndrome. Long-distance cycling is also a likely risk factor for vasculogenic and neurogenic ED (Huang et al, 2005). Several modifiable life style factors such as physical inability, smoking, and alcohol consumption may also contribute to ED (Kupelian et al, 2009).
Arterial disease is classified as extra- or intrapenile arterial insufficiency. Extrapenile disease may be amenable to surgical repair in selected patients, and comprises diseases of the internal pudendal artery, internal and common iliac arteries, aorta, the pelvic steal syndrome, and pelvic trauma. Intrapenile arterial disease secondary to aging, arteriosclerosis, or diabetes mellitus does not respond well to currently available surgical techniques.
Cavernous veno-occlusive dysfunction (CVOD) may result from a variety of pathophysiologic processes. Degenerative changes (Peyronie’s disease, aging, and diabetes) and traumatic injury to the tunica albuginea (penile fracture) can impair the compression of the subtunical and emissary veins. Fibroelastic alteration of the trabeculae, cavernous smooth muscle, and endothelium may result in venous leakage (Deveci et al, 2006). Men with diabetes mellitus and atherosclerosis are at increased risk of smooth-muscle atrophy, fibrous replacement, and endothelial disruption. In men with no symptoms of coronary disease, the presence of CVOD was reported to have the greatest chance of predicting abnormal exercise stress echocardiography (Mulhall et al, 2009).
CVOD can be divided into five types according to cause: In type 1, large veins exit the corpus cavernosum (etiology is likely congenital); in type 2, venous channels are enlarged as a result of distortion of the tunica albuginea (secondary to Peyronie’s disease or weakening associated with aging); in type 3, the cavernous smooth muscle is unable to relax because of fibrosis, degeneration; in type 4, there is inadequate neurotransmitter release (neurologic or psychologic impotence, or endothelial dysfunction) (Watts et al, 2007); and in type 5, there is abnormal communication between the corpus cavernosum and the spongiosum or glans (congenital, traumatic, or secondary to shunt procedures for priapism) (Dean and Lue, 2005).
Many drugs have been reported to cause ED, although the mechanism of action is often unknown and there are few controlled studies on the sexual side effects of a particular agent. As ED is common among older men, it will coexist with other conditions that are themselves risk factors for ED, such as cardiovascular disease, diabetes, or depression. Sexual symptoms related to medications can also involve a combination of complaints concerning desire, arousal, and orgasm rather than being limited to impaired erection.
In general, drugs that interfere with central neuroendocrine or local neurovascular control of penile smooth muscle have the potential to cause ED. Central neurotransmitter pathways, including serotonergic, noradrenergic, and dopaminergic pathways involved in sexual function, may be disturbed by antipsychotics, antidepressants, and centrally acting antihypertensive drugs (Balon, 2005). Selective serotonin reuptake inhibitors are the most common class of drugs currently used to treat depression; it is estimated that up to 50% of patients using these agents experience a change in sexual function. Nonspecific beta-adrenergic blocking drugs may cause ED by potentiating alpha-1 adrenergic activity in the penis. Conversely, alpha-1 blockers and angiotensin-II-receptor blockers both tend to improve sexual function during treatment and may therefore be useful when commencing antihypertensive therapy in men with preexisting ED. Thiazide diuretics have been reported to cause ED; spironolactone can also cause a decrease in libido and gynecomastia. Alpha-adrenergic blocking drugs, such as doxazosin, terazosin, and tamsulosin, may cause retrograde ejaculation owing to relaxation of the bladder neck (Guiliano, 2006). Other drugs thought to cause ED include opiates, antiretroviral agents, and histamine H2-receptor antagonists (cimetidine).
Antiandrogens modify sexual behavior by varying degrees, ranging from complete loss to normal function, chiefly by modulating sexual desire via central nervous system androgen receptors. Finasteride, a 5-alpha-reductase inhibitor commonly used to treat benign prostatic hypertrophy, is the antiandrogen with the least effect on circulating testosterone and sexual function. Sexual symptoms are reported in approximately 5% of men treated with a 5-mg dose (Miner et al, 2006). Estrogens and drugs with antiandrogenic action such as ketoconazole, luteinizing hormone-releasing hormone (LHRH) agonists, nonsteroidal (bicalutamide), and steroidal (cyproterone acetate) acetate can diminish sexual function. The near-complete androgen deprivation achieved by medical castration with LHRH agonists results in a profound loss of sexual desire, which is usually accompanied by ED.
Cigarette smoking may induce vasoconstriction and penile venous leakage because of its contractile effect on the cavernous smooth muscle and is seen to approximately double the rate of ED in coronary artery disease, hypertension, and atherosclerosis (Korenman, 2004). Alcohol in small amounts improves erection and increases libido because of its vasodilatory effect and the suppression of anxiety; however, large amounts can cause central sedation, decreased libido, and transient ED. Chronic alcoholism may cause hypogonadism and polyneuropathy, which may affect penile nerve function.
Sexual function progressively declines in “healthy” aging men. Longitudinal studies demonstrate a nonlinear decline for most aspects of sexual function as age increases, with a more pronounced decline in older groups (Araujo et al, 2004). The latent period between sexual stimulation and erection increases, erections are less turgid, ejaculation is less forceful, ejaculatory volume decreases, and the refractory period between erections lengthens. There is also a decrease in penile sensitivity to tactile stimulation, a decrease in serum testosterone concentration, and an increase in cavernous muscle tone. While psychologic and organic factors are important contributors to ED across age groups, organic issues tend to play a more profound role as men age.
ED in men with diabetes mellitus occurs approximately threefold that of the general population, and can be the presenting symptom for diabetes mellitus and/or predict later neurologic sequelae (Fonseca and Java, 2005). Diabetes may affect small vessels, cavernous nerve terminals, smooth muscle, and endothelial cells; neurovascular sequelae of long-term diabetes results in decreased responsiveness to oral PDE-5 inhibitor therapy. Obesity, a component of metabolic syndrome, has been linked to endothelial dysfunction, androgen deficiency, and ED (Traish et al, 2009). Alteration of protein levels in the corpus cavernousum has also been proposed as a cause of ED (Chiou et al, 2010).
Men with severe pulmonary disease may have ED because of fear of aggravating dyspnea during sexual intercourse (Koseoglu et al, 2005) Patients with angina, myocardial infarction (MI), or heart failure may have ED from anxiety, depression, or concomitant penile arterial insufficiency, which is quite common in these patients. Chronic renal failure has frequently been associated with diminished erectile function, impaired libido, and infertility (Shamsa et al, 2005). In men with chronic renal failure and ED, many were found to have cavernous artery occlusive disease and veno-occlusive dysfunction. The mechanism is likely multifactorial: low serum testosterone concentration, diabetes mellitus, vascular insufficiency, multiple medications, autonomic and somatic neuropathy, and psychological stress. Other systemic disorders such as cirrhosis, chronic debilitation, and cachexia can cause ED due to loss of libido or neurovascular dysfunction.
Diagnosis and Treatment
The management of ED is built on a patient-centered and evidence-based principle. A detailed medical, sexual, and psychosocial history and a thorough physical examination are the most important steps in the differential diagnosis of sexual dysfunction. Interviewing the partner, if available, is helpful in eliciting a reliable history, planning treatment, and obtaining a successful outcome.