Male Infertility: Introduction
Infertility is defined as the failure to conceive despite 1 year of regular unprotected intercourse. Approximately 15% of couples will experience infertility, and of these, 20% will have a male factor that is solely responsible; male factors will contribute in an additional 30% of cases. In general, male infertility is identified by abnormalities on a semen analysis; however, other issues can contribute to infertility despite normal semen.
The causes of male infertility are widely varied and are best evaluated by a urologist. Some causes of male infertility can be identified and reversed (or improved) with specific surgery or medication while other causes can be identified but not reversed. Occasionally, the underlying cause of infertility or an abnormal semen analysis cannot be identified, in which case it is termed idiopathic. These cases may be amenable to empiric treatment to improve the chances of conception. Before discussing the diagnosis and treatment of male infertility, a review of basic reproductive endocrinology, physiology, and anatomy is in order.
Male Reproductive Physiology
The hypothalamic–pituitary–gonadal (HPG) axis plays a critical role in both the endocrine (testosterone production) and exocrine (sperm maturation) functions of the testes. Several endocrine concepts must be reviewed.
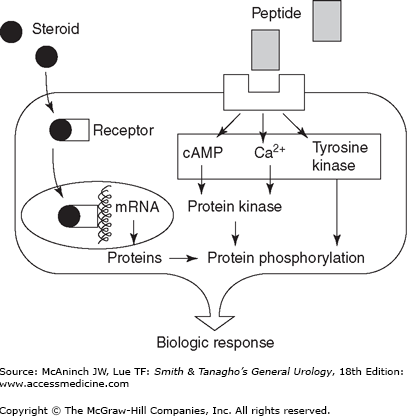
Both peptide and steroid hormones are required for communication in the reproductive axis. Peptide hormones are small secretory proteins that bind receptors on the cell surface membrane and induce a series of intracellular events. Hormone signals are transduced by second-messenger pathways whose actions culminate in the phosphorylation of various proteins that alter cell function. The key peptide hormones of the HPG axis are luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
Steroid hormones are derived from cholesterol and, unlike peptide hormones, are not stored in secretory granules. As a result, steroid secretion is limited by the rate of production. Since they are lipophilic, steroid hormones are generally cell membrane permeable. In plasma, steroid hormones are largely bound to serum proteins, with only a small “free” component available to diffuse into the intracellular space and bind receptors. Once bound to an intracellular receptor, steroids are translocated to deoxyribonucleic acid (DNA) recognition sites within the nucleus where they act by regulating the transcription of target genes. The key steroid hormones of the HPG axis are testosterone (T) and estradiol (E2).
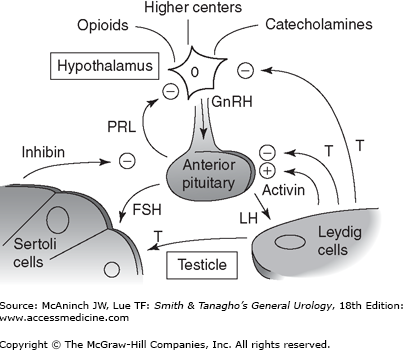
Normal endocrine and exocrine function of the testes depends on the orchestrated action of numerous hormones. Positive and negative feedback is the principal mechanism through which hormonal regulation occurs. By this mechanism, a hormone can regulate the synthesis and action of itself or of another hormone. Further coordination is provided by hormone action at multiple sites and through multiple responses. In the HPG axis, negative feedback is responsible for minimizing hormonal perturbations and maintaining homeostasis.
The hypothalamus receives and integrates neuronal input from the amygdala, thalamus, pons, retina, and cortex. The pulsatile secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus causes the cyclical secretion of pituitary and gonadal hormones. It is anatomically linked to the pituitary gland by both a portal vascular system and neuronal pathways, thus avoiding the systemic circulation. GnRH is a 10-amino acid peptide secreted from neurons in the preoptic and arcuate nuclei of the hypothalamus. Once secreted into the pituitary portal circulation, GnRH has a half-life of approximately 5–7 minutes, almost entirely removed on the first pass through the pituitary where it stimulates the secretion of FSH and LH.
GnRH secretion is highly responsive to a variety of hormonal and pharmacologic inputs (Table 44–1) and may also be impacted by stress, exercise, and diet. The pulse frequency of GnRH secretion varies from once or twice in 24 hours to every hour and can be abolished by the administration of GnRH agonists.
GnRH modulator | Type of feedback | Examples |
|---|---|---|
Opioids | Negative/inhibitory | β-Endorphin |
Catecholamines | Variable | Dopamine |
Peptide hormones | Negative/inhibitory | FSH, LH |
Sex steroids | Negative/inhibitory | Testosterone |
Prostaglandins | Positive/stimulatory | PGE2 |
The anterior pituitary gland is located within the sella turcica of skull and secretes a series of peptide hormones, including the gonadotropins. GnRH stimulates both production and release of FSH and LH by a calcium flux-dependent mechanism. The sensitivity of the pituitary gonadotrophs for GnRH varies with patient age and hormonal status.
LH and FSH are both glycoproteins composed of alpha and beta subunits, each coded by a separate gene. The alpha subunits of each hormone are identical and are similar to that of all other pituitary hormones; thus, their unique biologic activities are conferred by the beta subunits. Pulsatile secretion of LH varies from 8 to 16 pulses per day with variation in amplitude by one- to threefold. The pulse patterns reflect GnRH release and are regulated by androgens and estrogens through negative feedback. Pulsatile secretion of FSH occurs approximately every 1.5 hours and also demonstrates amplitude variation. Because FSH secretion is smaller in amplitude and has a longer serum half-life, its responsiveness to GnRH is more difficult to measure. In addition to its regulation by serum steroid hormones, FSH appears to have unique and independent responsiveness to the gonadal proteins inhibin and activin.
In the testis, LH stimulates steroidogenesis within Leydig cells by inducing the mitochondrial conversion of cholesterol to pregnenolone and testosterone. FSH binds to Sertoli cells and spermatogonial membranes within the testis and is the major stimulator of seminiferous tubule growth during development and initiates spermatogenesis at puberty. In adults, the major physiologic role of FSH is to maintain quantitatively normal spermatogenesis. Both FSH and LH bind cell surface receptors that activate adenylate cyclase and cause increases in intracellular cyclic adenosine monophosphate (cAMP).
Prolactin is also produced and secreted by the anterior pituitary and can impact both the HPG axis and fertility. Prolactin is a large (23 kDa), globular protein that potentiates milk production and lactation during pregnancy. The role of prolactin in men is poorly understood, but it may increase the concentration of LH receptors on the Leydig cell and may help sustain high intratesticular testosterone levels. It may also potentiate the effects of androgens on the growth and secretions of male accessory sex glands. Normal prolactin levels may be important in the maintenance of libido. Elevated prolactin appears to abolish gonadotropin pulsatility by interfering with episodic GnRH release. Importantly, a markedly elevated prolactin may be evidence of a prolactin-secreting adenoma of the pituitary (prolactinoma) that requires further evaluation.
Normal male reproduction requires that the testes have both endocrine (steroid production) and exocrine (sperm maturation and excretion) function. Both of these functions are under the control of the HPG axis. Steroidogenesis occurs in the interstitial compartment, where Leydig cells reside. Spermatogenesis occurs in the seminiferous tubules with the support of Sertoli cells.
Men normally produce approximately 5 g/day. Approximately 2% of testosterone circulates “free” in the serum and is considered the biologically active fraction. The remaining testosterone is bound to sex hormone–binding globulin (SHBG) and, to a slightly lesser extent, albumin within the blood. Several conditions can alter SHBG levels within the blood and thus changing the amount of free or bioavailable testosterone available for tissues. Elevated estrogens and thyroid hormone decrease plasma SHBG and increase the free testosterone fraction, whereas androgens, growth hormone, and obesity increase SHBG levels and decrease the active androgen fraction. Further, as men age, SHBG levels rise. Testosterone is a primary regulator of its own production through negative feedback on the HPG axis.
Testosterone is metabolized into two primary metabolites in target tissues: (1) dihydrotestosterone (DHT) by the enzyme 5-alpha-reductase and (2) estradiol by the enzyme aromatase. DHT is a more potent androgen than testosterone, and in many tissues, the conversion of testosterone to DHT is required for androgen action. While aromatase is present in many tissues, adipocytes play a significant role in the aromatization of testosterone to estradiol. While the mechanisms are still being elucidated, estradiol appears to have a pivotal role in the regulation of the HPG axis.
FSH acts primarily on Sertoli cells within the seminiferous tubules to induce the production of a number of proteins necessary for spermatogenesis, including androgen-binding protein, transferrin, lactate, ceruloplasmin, clusterin, plasminogen activator, prostaglandins, and several growth factors. Through these actions, seminiferous tubule growth is stimulated during development, and sperm production is initiated during puberty and maintained in adulthood.
Inhibin is a 32-kDa protein derived from Sertoli cells that inhibits FSH release from the anterior pituitary. Inhibin production is stimulated by FSH and acts by negative feedback at the pituitary and hypothalamus. Activin, a peptide hormone with structural homology to transforming growth factor-beta, appears to stimulate FSH secretion through its action on the hypothalamus and pituitary. Activin receptors are found in a host of extragonadal tissues, suggesting that this hormone may have a variety of growth factor or regulatory roles in the body.
Spermatogenesis
Spermatogenesis is a complex process during which primitive, multipotent stem cells divide to either renew themselves or produce daughter cells (mitosis) that further divide (meiosis) to become spermatozoa. These processes occur within the seminiferous tubules of the testis and 80–90% of testis volume is made up of the seminiferous tubules and germ cells at various developmental stages. Thus, it is not surprising that testicular hypotrophy and atrophy are strongly correlated to semen parameters.
Sertoli cells line the seminiferous tubules and are linked by tight junctions. These junctional complexes divide the seminiferous tubule space into basal (basement membrane) and adluminal (lumen) compartments and form the basis for the blood–testis barrier. As a result of this tight junction barrier, spermatogenesis occurs in an immunologically privileged site. Sanctuary from the immune system is critical given that spermatozoa are produced at puberty and therefore contain foreign antigens; immune recognition develops during the first year of life.
Sertoli cells foster spermatogenesis and participate in germ cell phagocytosis. FSH binding to high-affinity FSH receptors on Sertoli cells induces production and secretion of androgen-binding protein. Within the luminal fluid, this protein binds testosterone leading to levels 20–50 times that found in serum. Other regulatory effects of Sertoli cells include the production of inhibin and ligand-receptor complexes, such as c-kit and kit ligand.
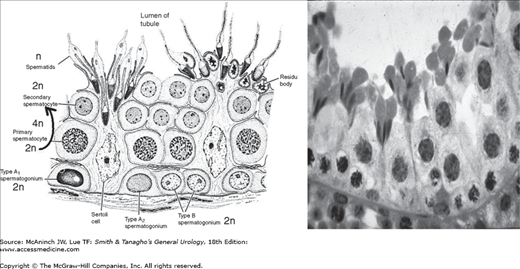
Germ cells are highly ordered within the cross section of the seminiferous tubules. Spermatogonia sit directly on the basement membrane and are followed by primary spermatocytes, secondary spermatocytes, and finally spermatids within the tubular lumen. Thirteen stages of germ cell differentiation have been identified in humans. The tight junctions of Sertoli cells support spermatogonia and early spermatocytes within the basal compartment while all subsequent stages of germ cell development occur within the adluminal compartment. Germ cells are staged by their histologic morphology; there are dark type A (Ad) and pale type A (Ap) and type B spermatogonia and preleptotene, leptotene, zygotene, and pachytene primary spermatocytes, secondary spermatocytes, and Sa, Sb, Sc, Sd1, and Sd2 spermatids (Figure 44–3).
Spermatogenesis is a cyclic process that involves the division of spermatogonial stem cells into elongated spermatids. Several individual cycles of spermatogenesis coexist within the germinal epithelium at a given time. In humans, an entire spermatogenic cycle requires approximately 60–80 days. Cohorts of developmentally similar germ cells are linked by cytoplasmic bridges and mature in unison. This cytoplasmic linkage of cells is organized in a spiral pattern along the seminiferous tubule and results in consistent and stable production of mature sperm.
Mitosis is the process by which somatic cells are replicated to form genetically identical daughter cells. Meiosis is the process by which germ cells replicate. As the result of meiosis, daughter cells called gametes are formed which contain one half the genetic material of the parent cell and thus allow for reproduction. This fundamental difference in cell replication generates genetic diversity through natural selection.
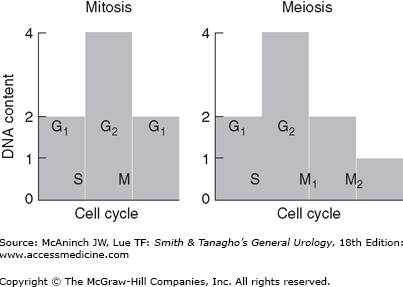
A cell’s life is divided into cycles that are each associated with different DNA replication activities. Only 5–10% of the cell cycle is spent in the mitotic phase (M), during which time genetic (DNA) and cellular division occur. Mitosis is a precise series of events that requires complete duplication of the genetic material (chromosomes), degeneration of the nuclear envelope, and equal division of chromosomes and cytoplasm into two daughter cells (Table 44–2). The difference between mitotic and meiotic replication is that in mitosis, a single DNA duplication is followed by one cell division; however, in meiosis, two-cell divisions take place to create four daughter cells (Figure 44–4). As a consequence, following meiosis, daughter cells (gametes) contain only half of the chromosome content of the parent cell and are said to be haploid (n) as opposed to diploid (2n). Other major differences between mitosis and meiosis are outlined in Table 44–3.
Mitotic phase | Cell cycle | Description of events |
|---|---|---|
Interphase | G1, S, G2 | DNA doubling occurs |
Prophase | M | Nuclear envelope degenerates; spindle forms |
Metaphase | M | Chromosomes align at cell equator |
Anaphase | M | Duplicated chromosomes separate |
Telophase | M | Chromosomes to poles, cytoplasm divides |
Mitosis | Meiosis |
|---|---|
Occurs in somatic cells | Occurs in sexual cycle cells |
One cell division, two daughter cells | Two cell divisions, four daughter cells |
Chromosome number maintained | Chromosome number halved |
No pairing, chromosome homologs | Synapse of homologs, prophase I |
No crossovers | >1 crossover per homolog pair |
Centromeres divide, anaphase | Centromeres divide, anaphase II |
Identical daughter genotype | Genetic variation in daughter cells |
From puberty onward, the process of spermatogenesis requires rapid and organized cell division, the likes of which are not seen in other cell lines of the human body. As a result, these highly specialized cells are produced in large quantities; up to 300 sperm per gram of testis tissue per second. Type B spermatogonia undergo mitosis to produce diploid primary spermatocytes (2n), which then duplicate their DNA during interphase. After the first meiotic division, each daughter cell contains a single partner of the homologous chromosome pair and are termed secondary spermatocytes (2n). During the second meiotic division, the chromatids separate at the centromere, yielding haploid spermatids (n).
Spermiogenesis is the process by which spermatids mature to become elongated spermatozoa within the basilar compartment of the seminiferous tubules. This process requires several weeks, and requires the following: (1) formation of the acrosome from the Golgi body, (2) formation of the flagellum from the centriole, (3) reorganization of the mitochondria around the midpiece, (4) extensive compaction of nuclear material, and (5) elimination of residual cytoplasm.
Many cellular elements contribute to the cellular morphologic changes during spermiogenesis, including chromosome structure, associated chromosomal proteins, the perinuclear cytoskeletal theca layer, the microtubules in the nucleus, subacrosomal actin, and Sertoli cell interactions. With completion of spermatid elongation, the Sertoli cell cytoplasm retracts around the developing sperm, stripping it of all unnecessary cytoplasm and extruding it into the tubule lumen. The mature sperm has remarkably little cytoplasm.
Testicular spermatozoa have limited or no motility and are therefore incapable of naturally fertilizing an egg (which has significant clinical implications). They become functional only after traversing the epididymis and where further maturation occurs. Anatomically, the epididymis is divided into three regions: caput or head, corpus or body, and cauda or tail. While the process of epididymal sperm maturation is still being elucidated, it is clear that several changes occur in sperm as they transit through the epididymis. These changes include alterations in cell membrane polarity, membrane protein composition, immunoreactivity, phospholipid and fatty acid content, and adenylate cyclase activity. A sperm’s transit time through the epididymis is approximately 10–15 days.
Fertilization
During natural conception, fertilization generally occurs within the ampullary portion of the fallopian tubes. Ovulation typically occurs during the middle of the female menstrual cycle and can be predicted by timing, change in body temperature, chemical detection of the luteinizing hormone surge, or by changes in cervical mucus. This mucus becomes more abundant and watery, thereby facilitating the entry of sperm into the uterus and protecting it from the acidic vaginal environment. Once within the female reproductive tract, sperm undergo physiologic changes referred to as capacitation.
Upon making contact with the egg, sperm undergo hyperactivation, characterized by profound changes in motility (large, lashing motions of the sperm tail). Lytic enzymes are released from the acrosome to allow penetration of the outer surface of the egg. Upon completion of the acrosome reaction, contact between sperm and egg is mediated by specific ligands and receptors on the surface of each gamete.
Diagnosis of Male Infertility
The purpose of the male infertility evaluation is to (1) identify and correct the reversible causes of male infertility with the goal of allowing a couple to conceive through intercourse, or with the least amount of technology; (2) identify irreversible conditions that may be amenable to treatment with assisted reproductive technology (ART) using the male partner’s sperm; (3) identify irreversible conditions in which the man’s sperm are not obtainable, in which case the couple may consider donated sperm or adoption; (4) identify medical diseases that may be associated with infertility and require treatment; and (5) identify specific genetic causes of infertility that may be transmitted to and impact offspring. Given that a male factor can be the cause of infertility in 30–40% of couples and is a contributing factor in 50% of cases, it is important to evaluate both partners in parallel. A complete urologic evaluation is important because male infertility may be the presenting symptom of otherwise occult but significant systemic disease. A complete evaluation must be comprehensive.
A man’s evaluation begins with a thorough reproductive, medical, and surgical history (Table 44–4). Important components of this history include (1) duration of infertility, coital timing and frequency, and sexual health; (2) prior paternity or fertility treatments; (3) childhood illnesses and development; (4) medical illnesses, prior infections, and medications; (5) prior surgeries or traumas; and (6) exposure to potential gonadal toxins, such as heat, radiation, chemical solvents, or pesticides.
Fertility history |
Previous pregnancies (all partners) |
Duration of infertility |
Previous infertility treatments |
Female evaluation |
Sexual history |
Erections |
Timing and frequency |
Lubricants |
Developmental history |
Cryptorchidism |
Childhood cancer/treatment |
Mumps orchitis |
Pubertal development |
Medical history |
Fevers |
Systemic illness—diabetes, cancer, infection |
Genetic diseases—cystic fibrosis, Klinefelter syndrome |
Surgical history |
Orchidopexy, cryptorchidism |
Herniorrhaphy |
Trauma, torsion |
Pelvic, bladder, or retroperitoneal surgery |
Transurethral resection for benign prostatic hyperplasia |
Pubertal onset |
Family history |
Cryptorchidism |
Midline defects (Kartagener syndrome) |
Hypospadias |
Exposure to diethylstilbestrol |
Other rare syndromes—prune belly, etc. |
Medications |
Testosterone |
Nitrofurantoin |
Cimetidine |
Sulfasalazine |
Spironolactone |
Alpha-blockers |
Social history |
Ethanol |
Smoking/tobacco |
Cocaine |
Anabolic steroids |
Occupational history |
Exposure to ionizing radiation |
Chronic heat exposure (saunas) |
Aniline dyes |
Pesticides |
Heavy metals (lead) |
A sexual history is important as many couples do not know how to precisely time intercourse to achieve a pregnancy. Following intercourse, sperm reside within the cervical mucus and crypts for 1–2 days and can survive longer; thus, an appropriate frequency of intercourse is every 2 days. Water-based lubricants such as K-Y Jelly, most skin lotions, and saliva can reduce sperm motility in vitro and should be avoided. If needed, acceptable lubricants include vegetable, safflower, and peanut oils as well as egg whites.
A general medical and surgical history is also important. Fever or acute infection can decrease testis function and semen quality. Given that spermatogenesis generally requires at least 60 days to complete, the impact of such insults may not be observable in the semen until 2 months after the event. Surgical procedures on the bladder, retroperitoneum, or pelvis can lead to infertility by causing ejaculatory dysfunction due to damage to the bladder neck, sympathetic nerves, or pelvic nerve plexus, respectively. Hernia surgery can result in obstruction of the vas deferens in 1% of cases; this incidence may be rising because of the recent increased use of highly inflammatory mesh patches.
Childhood diseases can have a significant impact on fertility. Severe unilateral orchitis occurs in approximately one-third of postpubertal mumps infections and bilateral orchitis in 10%. Mumps orchitis likely causes pressure necrosis of testis tissue due to severe edema with the later development of testis atrophy. Cryptorchidism is associated with decreased sperm production. This is true for both unilateral and bilateral cases. Longitudinal studies of affected boys have shown that abnormally low sperm counts can be found in 30% of men with unilateral cryptorchidism and 50% of men with bilateral undescended testes. While difficult to study from an epidemiologic standpoint, cryptorchidism appears to result in a higher risk of infertility. Similar to testicular cancer risk, early orchidopexy may reduce the risk of spermatogenic failure.
Exposure and medication histories are very relevant to fertility. Decreased sperm counts have been demonstrated in workers exposed to specific pesticides, which may alter normal testosterone/estrogen hormonal balance. Exposure to ionizing radiation can cause temporary reductions in sperm production with as little as 10 cGy and more permanent or severe reductions with higher doses. Several medications (Table 44–5) and ingestants such as tobacco, cocaine, and marijuana have all been implicated as gonadotoxins. The effects of these agents may be reversible on withdrawal. Anabolic steroids, often taken to increase muscle mass and development, act as contraceptives by inhibiting the pituitary–gonadal axis. Routine exposure to moist heat by use of hot tubs or saunas should be discouraged, as these activities can elevate intratesticular temperature and impair sperm production. Other characteristics associated with impaired fertility include obesity, electromagnetic radiation exposure (eg, significant exposure to high-voltage power lines, cell phones in the “on” position), occupational status (eg, dry cleaners, painters, farm workers), and job-related or other psychological stress.
Antihypertensive agents (Thiazides) |
Alpha-adrenergic blockers (Prazosin, Phentolamine) |
Antipsychotic agents |
Mellaril (thioridazine) |
Haldol (haloperidol) |
Librium |
Antidepressants |
Imipramine |
Amitriptyline |
The family and developmental histories may also provide clues to the infertility etiology. A family history of cystic fibrosis (CF), a condition associated with congenital absence of the vas deferens (CAVD), or intersex conditions is important. The existence of siblings with fertility problems may suggest that a Y chromosome microdeletion or a cytogenetic (karyotype) abnormality is present in the family. A history of delayed onset of puberty could suggest Kallmann or Klinefelter syndrome. A history of recurrent respiratory tract infections may suggest a ciliary defect characteristic of the immotile cilia syndromes. It is important to remember that reproductive technologies enable most men afflicted with such conditions to become fathers and therefore allow for the perpetuation of genetic abnormalities that may not be normally sustained.
A complete examination of the infertile male is important to identify general health issues associated with infertility. For example, the patient should be adequately virilized; signs of decreased body hair or gynecomastia may suggest androgen deficiency.
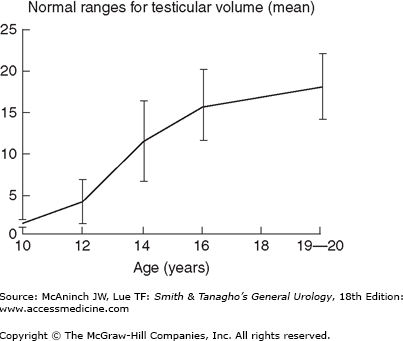
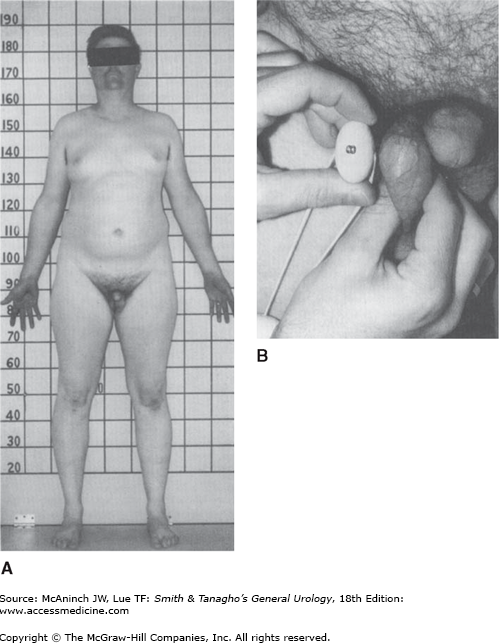
The scrotal contents should be carefully palpated with the patient standing. As it is often psychologically uncomfortable for young men to be examined, one helpful hint is to make the examination as efficient and matter of fact as possible. Two features should be noted about the testis: size and consistency. Size is assessed by measuring the long axis and width; as an alternative, an orchidometer can be placed next to the testis for volume determination (Figure 44–5). Standard values of testis size have been reported for normal men and include a mean testis length of 4.6 cm (range, 3.6–5.5 cm), a mean width of 2.6 cm (range, 2.1–3.2 cm), and a mean volume of 18.6 mL (± 4.6 mL) (Figure 44–6). Consistency is more difficult to assess but can be described as firm (normal) or soft (abnormal). Testes that are smaller than normal volume are termed hypotrophic, whereas those that are softer than normal are termed atrophic. Both conditions suggest impaired spermatogenesis.
Figure 44–6.
Normal values for testicular volume in relation to age. (Redrawn and reproduced, with permission, from Zachman M et al: Testicular volume during adolescence: Cross-sectional and longitudinal studies. Helv Paediatr Acta 1974;29:61; and McClure RD: Endocrine investigation and therapy. Urol Clin North Am 1987;14:471.)
The peritesticular area should also be examined. Irregularities of the epididymis, located posterior-lateral to the testis, include induration, tenderness, or cysts. The presence or absence of the scrotal vas deferens is critical to observe, as 2% of infertile men may present with CAVD.
Engorgement of the pampiniform plexus of veins in the scrotum is indicative of a varicocele. Asymmetry of the spermatic cords is the usual initial observation, followed by the feeling of a “bag of worms” when retrograde blood flow through the pampiniform veins occurs with a Valsalva maneuver. Varicoceles are usually found on the left side (90%) and are commonly associated with atrophy of the left testis. A discrepancy in testis size between the right and left sides should alert the clinician to this possibility.
Prostate or penile abnormalities should also be noted. Penile abnormalities such as hypospadias, abnormal curvature, or phimosis could result in inadequate delivery of semen to the upper vaginal vault during intercourse. Prostatic infection may be detected by the finding of a boggy, tender prostate on rectal examination. Prostate cancer, often suspected with unusual firmness or a nodule within the prostate, can occasionally be diagnosed in infertile men. Enlarged seminal vesicles, indicative of ejaculatory duct obstruction (EDO), may also be palpable on rectal examination.
Laboratory testing is an important part of the male infertility evaluation.
A carefully performed semen analysis is the primary source of information on sperm production and reproductive tract patency. However, it is not a measure of fertility. An abnormal semen analysis simply suggests the likelihood of decreased fertility. Studies have established that there are certain limits of adequacy below which it may be difficult to initiate a pregnancy. These semen analysis values were identified by the World Health Organization (2009) and are considered the minimum criteria for “normal” semen quality (Table 44–6). It is statistically more difficult to achieve a pregnancy if a semen parameter falls below any of those listed. Of these semen variables, the count and motility appear to correlate best with fertility.
Semen quality can vary widely in a normal individual from day to day, and semen analysis results are dependent on collection technique. For example, the period of sexual abstinence before sample collection is a large source of variability. With each day of abstinence (up to 1 week), semen volume can rise by up to 0.4 mL, and sperm concentration can increase by 10–15 million/mL. Sperm motility tends to fall when the abstinence period is longer than 5 days. For this reason, it is recommended that semen be collected after 48–72 hours of sexual abstinence.
To establish a baseline of semen quality, at least two semen samples are needed. Semen should be collected by self-stimulation; by coitus interruptus (less ideal); or with a special, nonspermicidal condom into a clean glass or plastic container. Because sperm motility decreases after ejaculation, the specimen should be analyzed within 1 hour of procurement. During transit, the specimen should be kept at body temperature.
Fresh semen is a coagulum that liquefies 15–30 minutes after ejaculation. Ejaculate volume should be at least 1.5 mL, as smaller volumes may not sufficiently buffer against vaginal acidity. Low ejaculate volume may indicate retrograde ejaculation, EDO, incomplete collection, or androgen deficiency. Sperm concentration should be >20 million sperm/mL. Sperm motility is assessed in two ways: the fraction of sperm that are moving and the quality of sperm movement (how fast, how straight they swim).
Sperm cytology or morphology is another measure of semen quality. By assessing the exact dimensions and shape characteristics of the sperm head, midpiece, and tail, sperm can be classified as “normal” or not. In the strictest classification system (Kruger morphology), only 14% of sperm in the ejaculate are normal looking. In fact, this number correlates with the success of egg fertilization in vitro and thus is ascribed real clinical significance. In addition, sperm morphology is a sensitive indicator of overall testicular health, because these characteristics are determined during spermatogenesis. The role of sperm morphology in the male infertility evaluation is to complement other information and to better estimate the chances of fertility.
In an effort to remove the subjective variables inherent in the manually performed semen analysis, computer-aided semen analyses (CASAs) couple video technology with digitalization and microchip processing to categorize sperm features by algorithms. Although the technology is promising, when manual semen analyses are compared with CASA on identical specimens, CASA can overestimate sperm counts by 30% with high levels of contaminating cells such as immature sperm or leukocytes. In addition, at high sperm concentrations, motility can be underestimated with CASA. CASA has accepted value in the research setting and in some clinical laboratories.
White blood cells (leukocytes) are present in all ejaculates and play important roles in immune surveillance and clearance of abnormal sperm. Leukocytospermia or pyospermia, an increase in leukocytes in the ejaculate, is defined as >1 × 106 leukocytes/mL semen but is not a significant cause of male subfertility and its treatment is debated. The prevalence of leukocytospermia ranges from 2.8% to 23% of infertile men. In general, neutrophils predominate among inflammatory cells (Table 44–7). This condition is detected by a variety of diagnostic assays, including differential stains (eg, Papanicolaou), peroxidase stain that detects the peroxidase enzyme in neutrophils, and immunocytology.
Antisperm antibodies (ASAs) can be found in three locations: serum, seminal plasma, and sperm bound. Among these, sperm-bound antibodies are the most relevant. The antibody classes that appear to be clinically relevant include immunoglobulin G (IgG) and IgA. IgG antibody is derived from local production and from transudation from the bloodstream (1%). IgA is thought to be purely locally derived.
Fructose is a carbohydrate that is secreted in high concentration from the seminal vesicles and is normally present in the ejaculate. When absent, seminal vesicle agenesis or obstruction may exist. Seminal fructose testing is indicated in men with low ejaculate volumes and no sperm. A postejaculate urinalysis inspects the first voided urine after ejaculation for the presence of sperm. If sperm is identified in the urine, a diagnosis of retrograde ejaculation is made. This test is indicated when the ejaculate volume is below normal (Table 44–8).
The testis is an immunologically privileged site due to the blood–testis barrier formed by the sertoli cell tight junctions. Autoimmune infertility may result when the blood–testis barrier is broken and the body is exposed to sperm antigens. Trauma to the testis and vasectomy are two common ways in which this occurs, giving rise to ASAs. ASA may be associated with impaired sperm transport through the reproductive tract or impairment in egg fertilization. An assay for ASA should be considered when (1) semen analyses show persistent sperm agglutination or clumping; (2) low sperm motility with history of testis injury or surgery; (3) idiopathic leukocytospermia; or (4) unexplained infertility.
Motility is the most commonly used measure of a sperm’s viability. Studies have suggested, however, that some nonmotile sperm may still be viable. Indeed, there are clinical conditions, such as immotile-cilia syndrome or testicular sperm, in which there may be immotile but otherwise healthy, viable sperm. Given that these sperm may be used in conjunction with intracytoplasmic sperm injection (ICSI) to form healthy embryos, it is clinically important that they are identifiable. Cell viability can be evaluated noninvasively by using the physiologic principle of hypoosmotic swelling. Viable cells with functional membranes swell when placed in a hypoosmotic environment. This response is easily observed in sperm as tail coiling generally accompanies head swelling. This sperm test is indicated in cases of complete absence of sperm motility.
It is possible to measure the ability of human sperm to penetrate a specially prepared hamster egg in the laboratory setting. The hamster egg allows interspecies fertilization but no further development. This form of bioassay can give important information about the ability of sperm to undergo the capacitation process as well as penetrate and fertilize the egg. Infertile sperm would be expected to penetrate and fertilize a lower fraction of eggs than normal sperm. The indications for the diagnostic sperm penetration assay (SPA) are limited to situations in which functional information about sperm is needed, that is, to further evaluate couples with unexplained infertility and to help couples decide whether intrauterine insemination (IUI) (good SPA result) or in vitro fertilization (IVF) and micromanipulation (poor SPA result) is the appropriate next treatment.
There is growing evidence that the integrity of sperm DNA is important for male fertility. Double- and single-stranded breaks in sperm DNA can be measured by several methods, including the COMET and TUNEL assays with or without the use of flow cytometry. These tests assess the degree of DNA fragmentation that occurs after chemically stressing the sperm DNA–chromatin complex, and can indirectly reflect the quality of sperm DNA–chromatin complex and can indirectly reflect the quality of sperm DNA integrity. Abnormally fragmented sperm DNA rarely occurs in fertile men, but can be found in a larger percentage of infertile men with otherwise normal semen analyses. This test can detect infertility that is missed on a conventional semen analysis. Often reversible, causes of DNA fragmentation include tobacco use, medical disease, hyperthermia, air pollution, infections, and varicocele.
Evaluation of the pituitary–gonadal axis provides valuable information on the state of sperm production. Furthermore, abnormal function of the HPG axis can be an underlying cause of poor sperm production and infertility (hyperprolactinemia, gonadotropin deficiency, congenital adrenal hyperplasia). In general, FSH and testosterone should be measured in infertile men with sperm concentrations of <10 × 106 sperm/mL. Testosterone is a measure of overall endocrine balance and likely plays a critical role in spermatogenesis. In most cases, FSH reflects the state of sperm production: when spermatogenesis is impaired at the testicular level, FSH should be elevated. This combination of tests will detect most (99%) endocrine abnormalities. Serum LH and prolactin levels may be obtained if testosterone and FSH are abnormal. In the setting of low testosterone, free testosterone should be assessed. Given the role of estradiol in regulating the HPG axis, it too should be assessed if there is evidence of poor virilization or obesity. Thyroid hormone, liver function, and other organ-specific tests should be obtained if there is clinical evidence of active disease, as uncontrolled systemic illness can affect sperm production. The common patterns of hormonal disorders observed in infertility are given in Table 44–9.
Condition | T | FSH | LH | PRL |
|---|---|---|---|---|
Normal | NL | NL | NL | NL |
Primary testis failure | Low | High | NL/High | NL |
Hypogonadotropic hypogonadism | Low | Low | Low | NL |
Hyperprolactinemia | Low | Low/NL | Low | High |
Androgen resistance | High | High | High | NL |
With relatively normal spermatogenesis, low levels of plasma LH and FSH have no clinical meaning; likewise, an isolated low LH with normal testosterone is not significant. Additional indications for hormone evaluation of infertile men include impaired sexual function (low libido, erectile dysfunction) and findings suggestive of a specific endocrinopathy (eg, thyroid). On initial testing, approximately 10% of infertile men will have an abnormal hormone level, with clinically significant endocrinopathies occurring in 2% of men.
Subtle genetic abnormalities can present as male infertility. It is estimated that between 2% and 15% of infertile men with azoospermia (no sperm count) or severe oligospermia (low sperm counts) will harbor a chromosomal abnormality on either the sex chromosomes or autosomes. A blood test for cytogenetic analysis (karyotype) can determine if such a genetic anomaly is present. Patients at risk for abnormal cytogenetic findings include men with small, atrophic testes; elevated FSH values; and azoospermia. Klinefelter syndrome (XXY) is the most frequently detected sex chromosomal abnormality among infertile men (Figure 44–7).