Lymphoproliferative Disorders of the Gastrointestinal Tract
In this discussion of gastrointestinal lymphoproliferative disorders the emphasis will be on neoplastic diseases. The lymphoid hyperplasias will be considered only in the context of their differential diagnosis from lymphomas. Being the most common site of primary extranodal lymphoma, the gastrointestinal tract accounts for between 30% and 50% of all cases (1,2). These lymphomas are almost exclusively of non-Hodgkin type; primary gastrointestinal Hodgkin lymphoma, although well documented, is vanishingly rare. In the West, gastrointestinal lymphoma comprises between 4% and 18% of all non-Hodgkin lymphomas, but there is some evidence that the incidence is rising (3,4). There is considerable geographic variation in the incidence of primary gastrointestinal lymphoma best illustrated by the high incidence in the Middle East. Exact figures are difficult to obtain but in the Middle East, excluding skin tumors, lymphoma as a whole is the most common malignancy, and 25% of these lymphomas arise primarily in the gastrointestinal tract (5). Considerable differences in incidence also exist between Western countries; there is, for example, a 13-fold higher incidence of primary gastric lymphoma in northeastern Italy compared to Britain (6).
Definition
Nodal lymphoma frequently involves the gastrointestinal tract as a secondary phenomenon; this frequency has almost certainly been underestimated as shown by the study of Fischbach et al (7), who performed gastroscopies on newly diagnosed nodal lymphomas and found gastric involvement in over 25%, including cases of low and high histologic grade lymphoma. Strict criteria for a diagnosis of primary gastrointestinal lymphoma are, therefore, necessary if its incidence is not to be overestimated. The criteria laid down by Dawson et al in 1961 (8), which require that the lymphoma be limited to the gastrointestinal tract and its contiguous lymph nodes, are still applicable, although they do not take account of modern staging procedures, which can detect small foci of disease in the liver and bone marrow, the presence of which does not necessarily exclude a primary gastrointestinal tumor. An operational definition of primary gastrointestinal lymphoma is that lymphoma presents with the main bulk of disease in the gastrointestinal tract necessitating the direction of treatment primarily to that site. Inevitably, this definition is blurred at its edges, and some widely disseminated gastrointestinal lymphomas will not be included while occasional cases of selectively disseminated nodal lymphomas might be wrongly assigned.
Sites of Origin
The stomach is by far the most common site of primary gastrointestinal lymphoma in the West and Far East (9,10), followed by the small intestine. The opposite is true in the Middle East (5), where most arise in the small intestine and the stomach is next in frequency. In all regions, colonic, rectal, and esophageal lymphomas account for a very small minority of cases. The distribution of gastrointestinal tract lymphomas is paradoxic since the normal gastric mucosa is almost devoid of lymphoid tissue and many primary intestinal lymphomas arise proximal to the terminal ileum, where there is the greatest concentration of mucosa (gut)-associated lymphoid tissue (MALT) in the form of Peyer patches.
Staging
The Musshoff modification of the Ann Arbor staging system for extranodal lymphoma (11) is most commonly used for staging gastrointestinal lymphomas. In stage IE (where E signifies an extranodal site), the lymphoma is confined to the wall of the stomach or intestine. In stage II1E, there is involvement of regional lymph nodes that are contiguous with the primary site, while in stage II2E, there is involvement of a regional but noncontiguous lymph node group. Stage III refers to involvement of lymph nodes on both sides of the diaphragm, the spleen (stage IIIS), or both (stage IIIE+S). In stage IV, there is dissemination to the bone marrow or other nonlymphoid organs.
Classification of Primary Gastrointestinal Lymphoma
With the few exceptions of other “organ-specific” lymphomas such as certain cutaneous T-cell lymphomas, splenic
marginal zone lymphoma, and some other rare entities, most lymphomas listed in the World Health Organization (WHO) lymphoma classification (12) can arise in the gastrointestinal tract. In terms of frequency, however, the types of lymphomas occurring in lymph nodes and the gastrointestinal tract are quite different, and there are also certain gastrointestinal lymphomas that do not occur in peripheral lymph nodes. A classification of primary gastrointestinal lymphomas is given in Table 18.1.
marginal zone lymphoma, and some other rare entities, most lymphomas listed in the World Health Organization (WHO) lymphoma classification (12) can arise in the gastrointestinal tract. In terms of frequency, however, the types of lymphomas occurring in lymph nodes and the gastrointestinal tract are quite different, and there are also certain gastrointestinal lymphomas that do not occur in peripheral lymph nodes. A classification of primary gastrointestinal lymphomas is given in Table 18.1.
TABLE 18.1 Primary Gastrointestinal Non-Hodgkin Lymphoma | ||||||
|---|---|---|---|---|---|---|
|
B-cell Lymphomas
Amongst B-cell lymphomas, MALT lymphoma, of which gastric MALT lymphoma is the prototype and most intensively studied, is the most interesting, if not the most common gastrointestinal lymphoma. Immunoproliferative small intestinal disease (IPSID) is a specific subtype of MALT lymphoma distinguished by its epidemiology and association with the synthesis of an abnormal α heavy chain (13,14). MALT lymphoma, including IPSID, may undergo transformation into diffuse large B-cell lymphoma (DLBCL) with loss of the characteristic histologic hallmarks of the original disease, but in at least a proportion of gastrointestinal DLBCL, careful examination will reveal residual foci of MALT lymphoma (15). However, most gastrointestinal DLBCLs, which comprise the majority of gastrointestinal lymphomas, appear to have arisen de novo without any evidence for transformation from a MALT lymphoma, and some of these express antigens such as CD10, which clearly indicates that they are not related to MALT lymphoma. Other B-cell tumors that arise in the gastrointestinal tract include mantle cell lymphoma, which often results in the condition called lymphomatous polyposis (16); a variant of follicular lymphoma; and Burkitt lymphoma, the latter being particularly common in the Middle East (17). Immunodeficiency-associated lymphoproliferative disorders and lymphomas also tend to arise primarily in the gastrointestinal tract. Finally, any type of B-cell lymphoma other than those listed above may present primarily in the gastrointestinal tract although not necessarily arising there.
T-cell and NK-cell Lymphomas
Primary gastrointestinal T-cell lymphomas are much less common than B-cell tumors. Enteropathy associated T-cell lymphoma (EATL) (18,19), which occurs as a complication of celiac disease, is the only distinctive T-cell tumor of the intestine and, in some ways, is the equivalent of B-cell MALT lymphoma in that it appears to arise from a gut-committed T-cell. Other T-cell lymphomas less commonly arise in the gastrointestinal tract.
NK-cell lymphomas of nasal type, although, as their name suggests, are more common in the upper respiratory tract, not infrequently present as primary intestinal tumors (20).
A number of other tumors of hematopoietic tissue, including histiocytic neoplasms (21) and granulocytic sarcoma (22), may also occur as primary gastrointestinal tumors. Although these are, strictly speaking, not lymphomas, they are easily mistaken for lymphoid tumors and are included in the WHO lymphoma classification.
Malt Lymphoma
In formulating classifications of non-Hodgkin lymphomas, considerable attention has been paid to architectural, cytologic, and functional similarities between the various lymphomas and normal lymphoid tissue as exemplified by the peripheral lymph node. However, studies of extranodal lymphomas, particularly gastrointestinal lymphomas, have suggested that their clinicopathologic features are not related to lymph nodes but instead to the structure and function of MALT (23,24).
The anatomic distribution and structure of lymph nodes are adapted to deal with antigens carried to the node in afferent lymphatics, which drain sites at various distances from the node. Permeable mucosal sites, such as the gastrointestinal tract, however, are particularly vulnerable to pathogens and antigens since they are in direct contact with the external environment, and specialized lymphoid tissue has evolved to protect them. This MALT includes gut-associated lymphoid tissue, nasopharyngeal lymphoid tissue (the tonsils), and other less well-characterized aggregates of lymphoid tissue related to other mucosae. Gut-associated lymphoid tissue serves as the paradigm for MALT.
Histology and Immunology of MALT
MALT in the gastrointestinal tract consists of four lymphoid compartments that include organized collections of lymphoid tissue, which, when concentrated in the terminal ileum, form Peyer patches; the lamina propria lymphocytes; plasma cells and accessory cells; intraepithelial lymphocytes; and the mesenteric lymph nodes. MALT lymphomas essentially recapitulate the features of Peyer patches.
Peyer Patches
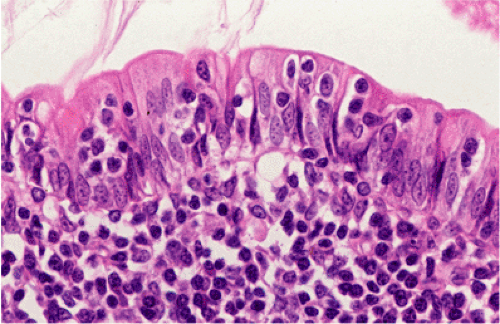
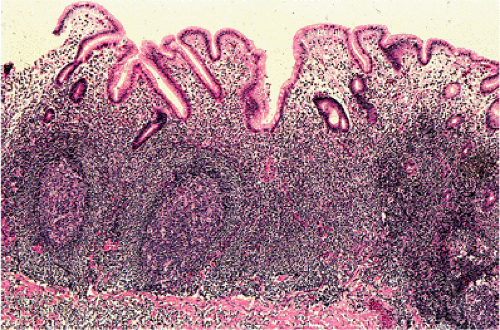
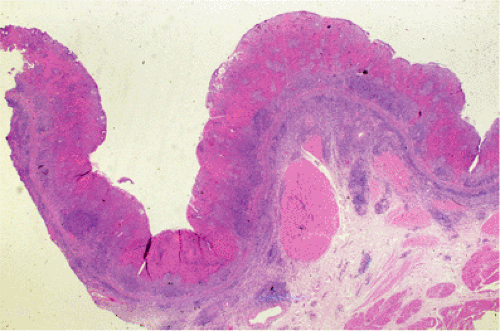
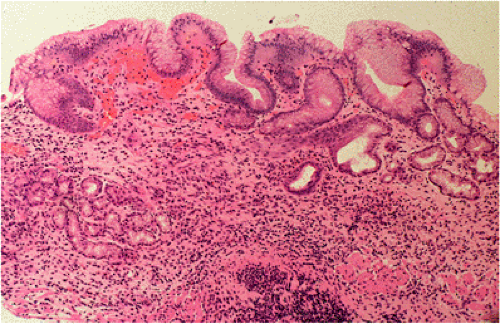
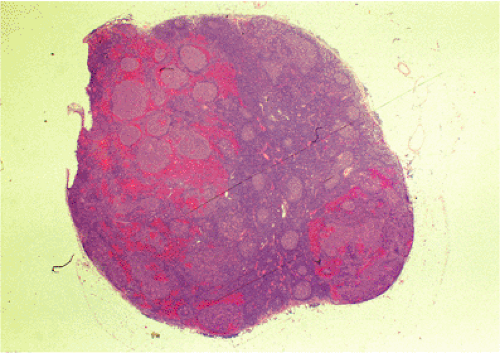
Organized lymphoid nodules are distributed throughout the small intestine, the appendix, and the colorectum. These nodules concentrate in the terminal ileum, where they collectively form the Peyer patches, the generic term applied to this compartment of MALT. Peyer patches are unencapsulated aggregates of lymphoid cells that bear a certain resemblance to lymph nodes (Fig. 18.1). Each Peyer patch nodule consists of B- and T-cell areas and associated accessory cells. The B-cell area is composed of a germinal center surrounded by a mantle zone of small B lymphocytes, which is broadest at the mucosal aspect of the follicle. Surrounding the mantle zone is a broad marginal zone in which most of the cells are small to intermediate-sized B lymphocytes with moderately abundant, pale-staining cytoplasm and nuclei with a slightly irregular outline leading to a resemblance to centrocytes. The marginal zone extends toward the mucosal surface and some marginal zone B cells enter the overlying dome epithelium, where they form the lymphoepithelium, which is a defining feature of MALT (Fig. 18.2). Immunohistochemical studies of Peyer patches (25,26,27) have shown that the B-cell follicles are identical to those of lymph nodes. In contrast to the IgM- and IgD-positive mantle zone, the peripheral marginal zone cells are IgM positive but IgD negative. Lateral to the deep aspect of the B-cell follicle, there is a T-cell zone in which high endothelial venules are prominent, equivalent to the paracortical T zone of the lymph node.
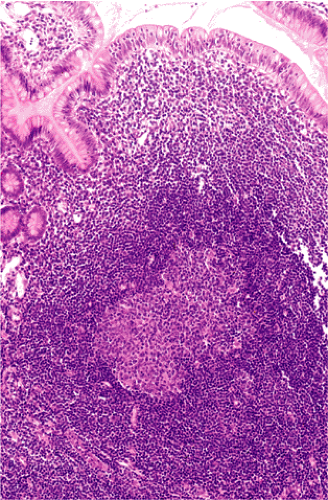 FIG. 18.1. Normal Peyer patch. A B-cell follicle with a subepithelial marginal zone covered by the dome epithelium. |
Definition of MALT Lymphoma
MALT lymphoma is listed in the WHO classification (12) under the designation “extranodal marginal zone lymphoma of mucosa associated lymphoid tissue” (MALT lymphoma) and defined as a lymphoma that recapitulates the histology of MALT (Peyer patches); the normal cell counterpart is the marginal zone B cell. MALT lymphomas arise in MALT acquired following chronic inflammation (acquired MALT) and consequently their clinical and pathologic features merge with those of the preceding chronic inflammatory disorder. MALT lymphomas consist of morphologically heterogeneous small B cells including marginal zone (centrocytelike) cells, cells resembling monocytoid cells, small lymphocytes, and scattered immunoblast and centroblastlike cells. There is plasma cell differentiation in a proportion of the cases. The infiltrate appears to arise in the marginal zone of reactive B-cell follicles, which, although reactive, are an integral part of MALT lymphomas and extends into the interfollicular region. In epithelial tissues the neoplastic cells typically infiltrate the epithelium, forming lymphoepithelial lesions (12).
Epidemiology
MALT lymphoma comprises 7% to 8% of all B-cell lymphomas and at least 50% of primary gastric lymphomas
(28,29). Most cases occur in adults with a median age of 61 years and a slight female predominance. There is a higher incidence of gastric MALT lymphoma in northeastern Italy, probably related to a high prevalence of Helicobacter pylori–associated gastritis in that region (6). Histologically, identical intestinal (30,31) and esophageal lymphomas also occur, but they are distinctly infrequent in comparison. A special subtype of small intestinal MALT lymphomas known as immunoproliferative small intestinal disease occurs in the Middle East, parts of the Indian subcontinent, and the Cape region of South Africa (14).
(28,29). Most cases occur in adults with a median age of 61 years and a slight female predominance. There is a higher incidence of gastric MALT lymphoma in northeastern Italy, probably related to a high prevalence of Helicobacter pylori–associated gastritis in that region (6). Histologically, identical intestinal (30,31) and esophageal lymphomas also occur, but they are distinctly infrequent in comparison. A special subtype of small intestinal MALT lymphomas known as immunoproliferative small intestinal disease occurs in the Middle East, parts of the Indian subcontinent, and the Cape region of South Africa (14).
Etiology
MALT lymphomas only rarely arise from native MALT; they more usually arise from MALT that has been acquired as a result of a chronic inflammatory disorder at sites normally devoid of MALT, including the stomach (Fig. 18.3). Here MALT is commonly acquired almost always as a result of the reaction to infection with H. pylori, which precedes development of most cases of gastric MALT lymphoma (Fig. 18.4) (32). A similar relationship has been proposed between intestinal infection with Campylobacter jejuni and IPSID (33). The functional characteristics of acquired MALT and the degree to which it resembles normal MALT have not been investigated. Likewise, the factors that, in a small number of cases, result in transformation of this reactive MALT into a lymphoma that recapitulates many of its normal morphologic and functional properties remain speculative.
Helicobacter pylori and Gastric MALT Lymphoma
Several lines of evidence suggest that gastric MALT lymphoma arises from MALT acquired as a consequence of H. pylori infection. H. pylori can be demonstrated in the gastric mucosa of the majority of cases of gastric MALT lymphoma (Fig. 18.5) (34). The first study in which this association was examined showed that the organism was present in over 90% of cases. Subsequent studies have shown a lower incidence (35) but also that the density and detectability of H. pylori decreases as lymphoma evolves from chronic gastritis (36). A subsequent case control study showed an association between previous H. pylori infection and the development of primary gastric lymphoma (37). More compelling evidence confirming a role for H. pylori in the pathogenesis of gastric lymphoma has been obtained from studies that detected the lymphoma B-cell clone in the chronic gastritis that preceded the lymphoma (36) and from a series of in vitro studies showing that lymphoma growth could be stimulated in culture by H. pylori strain–specific T cells when crude lymphoma cultures were exposed to the organism (38). Finally, following the initial study by Wotherspoon et al (39), several groups have confirmed that eradication of H. pylori with antibiotics results in regression of gastric MALT lymphoma in 75% of cases (40) (see below).
Campylobacter jejuni and Immunoproliferative Small Intestinal Disease
Unlike gastric MALT lymphomas, relatively few cases of IPSID, which is in any case rare, have definitively been shown to respond to broad-spectrum antibiotics. Moreover, the presumptive organism linked to IPSID has remained
unknown. In 2004, Lecuit et al (33), based on a single case report, suggested that C. jejuni may play the same role in IPSID as H. pylori does in gastric MALT lymphoma. Isaacson et al (unpublished) in a polymerase chain reaction (PCR) study confirmed an association between C. jejuni and IPSID but also detected the organism in other small intestinal lymphomas. To date, no laboratory study on the effects of C. jejuni on IPSID cells has been reported, and further studies on the effects of C. jejuni eradication are awaited.
unknown. In 2004, Lecuit et al (33), based on a single case report, suggested that C. jejuni may play the same role in IPSID as H. pylori does in gastric MALT lymphoma. Isaacson et al (unpublished) in a polymerase chain reaction (PCR) study confirmed an association between C. jejuni and IPSID but also detected the organism in other small intestinal lymphomas. To date, no laboratory study on the effects of C. jejuni on IPSID cells has been reported, and further studies on the effects of C. jejuni eradication are awaited.
Histology of Acquired Mucosa-associated Lymphoid Tissue
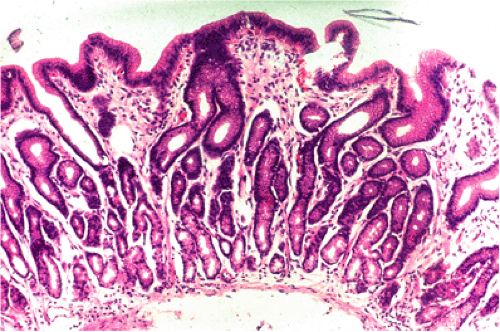
Tissues in which MALT lymphomas occur seem to mount a stereotyped response to certain known and unknown agents with accumulation of lymphoid tissue that forms Peyer patch–like structures (Fig. 18.4).
Because of its unique ability to withstand a low pH, H. pylori is the one organism, apart from some other, rare Helicobacter strains, that can survive in the human gastric mucosa. The prevalence of H. pylori gastritis in any given population varies from 20% to 100% depending on the locality and the age cohort (see Chapter 4). With some exceptions, the prevalence of gastric MALT lymphoma is related to that of H. pylori gastritis. Typically, infection results in active chronic inflammation with B-cell follicles and the formation of a lymphoepithelium by B-cell infiltration of glands immediately adjacent to the follicles (41) (Fig. 18.4)—features of acquired MALT. Between the follicles, the gastric mucosal lamina propria is infiltrated by T lymphocytes, plasma cells, macrophages, and occasional collections of neutrophils. The lymphoid infiltrate may be extremely florid and at times difficult to distinguish from MALT lymphoma, especially when there are expanded sheets of mantle zone cells in biopsy fragments.
Immunohistochemistry is useful in delineating the B-cell follicles and distinguishing the IgM- and IgD-positive mantle zone cells from IgM-positive, IgD-negative MALT lymphoma cells. Staining for immunoglobulin light chains can be useful in detecting monoclonal B cells and plasma cells in some cases of MALT lymphoma; however, the presence of polyclonal plasma cells does not exclude the diagnosis. PCR analysis normally reveals a polyclonal B-cell population in gastritis, but there are reports of spurious monoclonality detected in gastric biopsies from patients with H. pylori gastritis (42). When the test is properly performed and interpreted this is extremely uncommon (43), but it is noteworthy that in patients with gastritis who have later developed MALT lymphoma, the identical monoclonal B-cell population has been detected in both lesions (36).
Clinical Presentation
Patients with gastric lymphoma usually present with nonspecific dyspepsia; severe abdominal pain and the presence of an abdominal mass are rare. The findings at endoscopy are usually those of nonspecific gastritis and/or a peptic ulcer, the presence of a mass again being unusual. Most gastric
MALT lymphomas are at stage IE at the time of diagnosis. Gastric lymph node involvement (stage I1E) is present in 5% to 10% and bone marrow involvement (stage IVE) may be found in up to 10% of cases (44).
MALT lymphomas are at stage IE at the time of diagnosis. Gastric lymph node involvement (stage I1E) is present in 5% to 10% and bone marrow involvement (stage IVE) may be found in up to 10% of cases (44).
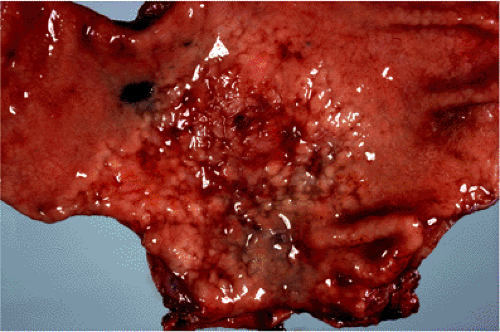 FIG. 18.6. Gastric mucosa-associated lymphoid tissue lymphoma resulting in a “cobblestone” appearance of the gastric mucosa. |
Pathology of MALT Lymphoma
Gross Features
Macroscopically, MALT lymphomas, although sometimes forming obviously tumorous masses, frequently are indistinguishable from the inflammatory lesion that underlies the acquisition of MALT from which the lymphoma arises. Gastric MALT lymphoma, for example, may form a single dominant mass but often results in only slightly raised congested mucosa with superficial erosions easily confused at endoscopy with chronic gastritis (Fig. 18.6). MALT lymphomas are typically multifocal with small, even microscopic foci of lymphoma scattered throughout the organ involved. Each of these foci is clonally identical (45).
Histopathology
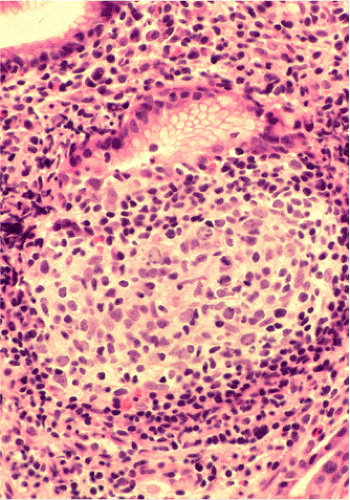
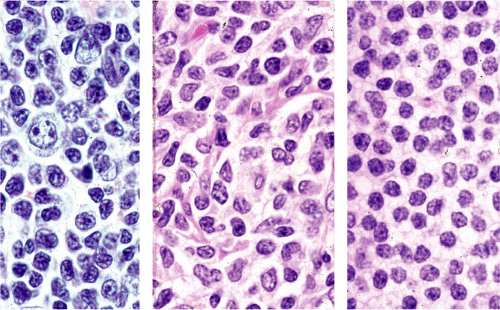
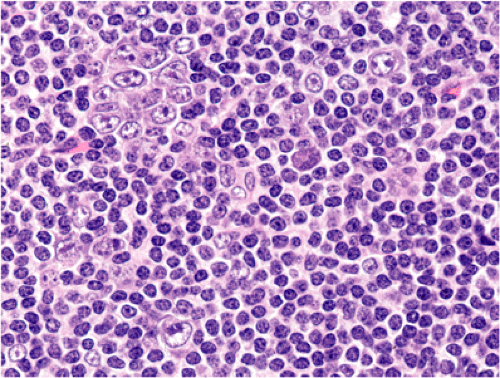
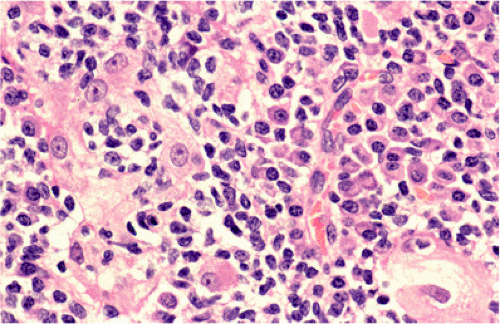
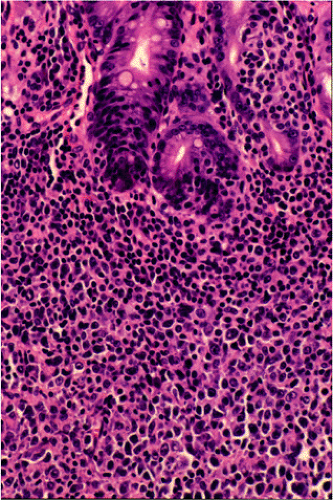
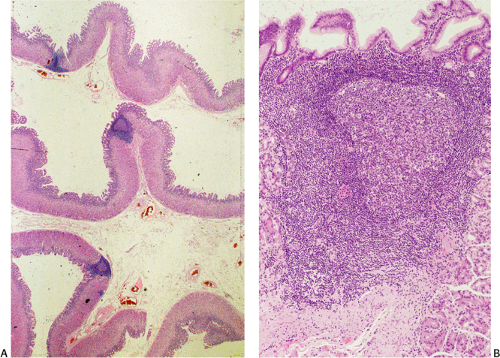
Although there are some differences determined by the site of origin, the histology of MALT lymphoma is essentially stereotyped in that, like acquired MALT, the lymphomas, especially in their early stages, recapitulate the histology of Peyer patches (46). The neoplastic B lymphocytes infiltrate around reactive B-cell follicles, external to a preserved follicular mantle, in a marginal zone distribution and spread out to form larger confluent areas, which eventually overrun some or most of the follicles (Fig. 18.7). Like marginal zone B cells, the neoplastic cells have pale cytoplasm with small to medium-sized, slightly irregularly shaped nuclei containing moderately dispersed chromatin and inconspicuous nucleoli. These cells have been called centrocyte-like because of their resemblance to germinal center centrocytes. The accumulation of more abundant pale-staining cytoplasm may lead to a monocytoid appearance of the lymphoma cells, while in some cases the cells more closely resemble small lymphocytes (Fig. 18.8). Scattered large cells resembling centroblasts or immunoblasts are usually present, but are in the minority and do not form confluent clusters or sheets (Fig. 18.9). Plasma cell differentiation is present in up to a third of cases (Fig. 18.10) and, in gastric lymphomas, tends to be maximal beneath the surface gastric epithelium. In a subset of cases there is extreme plasma cell differentiation characterized by a subepithelial band of confluent large eosinophilic plasma cells often accompanied by lakes of extruded immunoglobulin (Fig. 18.11). Small clusters of neoplastic marginal zone cells are present often invading individual gastric glands to form lymphoepithelial lesions (Fig. 18.12). In these lesions, glandular epithelium is invaded and destroyed by discrete aggregates of lymphoma cells (Fig. 18.13). Lymphoepithelial lesions are defined as aggregates of three or more neoplastic marginal zone lymphocytes within glandular epithelium preferably associated with distortion or necrosis of the epithelium. In gastric MALT lymphoma the lesions are often accompanied by eosinophilic degeneration of the epithelium (Fig. 18.13). Lymphoepithelial lesions, although highly characteristic of MALT lymphoma, especially gastric lymphoma, are not pathognomonic. In some MALT lymphomas, such as those of the small and large intestine, they are difficult to find.
The MALT lymphoma cells sometimes specifically colonize germinal centers of the reactive follicles (47). Usually this imparts a vaguely nodular or follicular pattern to the lymphoma (Fig. 18.14). In some cases the lymphoma cells specifically target germinal centers, where they may undergo blast transformation (Fig. 18.15) or plasma cell differentiation (Fig. 18.16). The presence of transformed blasts confined to pre-existing germinal centers is not considered to be evidence of transformation to a large B-cell lymphoma.
Like other low-grade B-cell lymphomas, MALT lymphoma may undergo transformation to a diffuse large B-cell lymphoma (15). Transformed centroblast or immunoblastlike
cells are present in variable numbers in MALT lymphoma (Fig. 18.9) and there is some evidence that grading of MALT lymphoma according to the number of transformed cells has subtle clinical relevance (48). However, only when solid or sheetlike proliferations of transformed cells are present should the lymphoma be considered to have transformed to diffuse large B-cell lymphoma (Fig. 18.17). This transformation may or may not result in complete overgrowth of the preceding MALT lymphoma. The current recommendation is that such cases are best designated as diffuse large B-cell lymphoma; the presence or absence of concurrent MALT lymphoma and the relative proportions of both should be documented (49).
cells are present in variable numbers in MALT lymphoma (Fig. 18.9) and there is some evidence that grading of MALT lymphoma according to the number of transformed cells has subtle clinical relevance (48). However, only when solid or sheetlike proliferations of transformed cells are present should the lymphoma be considered to have transformed to diffuse large B-cell lymphoma (Fig. 18.17). This transformation may or may not result in complete overgrowth of the preceding MALT lymphoma. The current recommendation is that such cases are best designated as diffuse large B-cell lymphoma; the presence or absence of concurrent MALT lymphoma and the relative proportions of both should be documented (49).
Biopsy Appearances
In small endoscopic biopsies the classic features of MALT lymphoma are not as easily observed as they are in resection
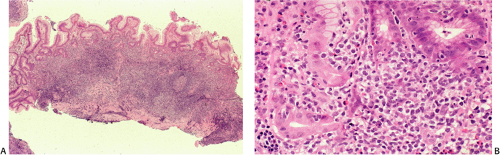
specimens. Reactive follicles are not as easy to define and are particularly affected by crush artefact. Equally, the cytologic appearances of marginal zone cells may not be as clear. A diffuse, dense lymphoid infiltrate in a gastric biopsy (Fig. 18.18A) should always raise suspicion, particularly if it occupies the entire biopsy fragment. Careful evaluation of the cytology of the cells is indicated (Fig. 18.18B) and identification of lymphoepithelial lesions (Fig. 18.18B) is very helpful in establishing the diagnosis.
specimens. Reactive follicles are not as easy to define and are particularly affected by crush artefact. Equally, the cytologic appearances of marginal zone cells may not be as clear. A diffuse, dense lymphoid infiltrate in a gastric biopsy (Fig. 18.18A) should always raise suspicion, particularly if it occupies the entire biopsy fragment. Careful evaluation of the cytology of the cells is indicated (Fig. 18.18B) and identification of lymphoepithelial lesions (Fig. 18.18B) is very helpful in establishing the diagnosis.
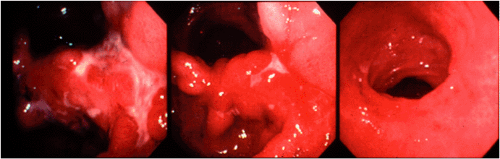
Morphology of Gastric Mucosa-associated Lymphoid Tissue Lymphoma Following Eradication of Helicobacter pylori
Approximately 75% of gastric MALT lymphomas will respond to eradication of H. pylori with regression of the tumor over a period of up to 18 months (Fig. 18.19) (39). Repeated endoscopy with biopsy is necessary to determine whether or not the lymphoma is responding. The endoscopic appearances may revert within 6 months of the eradication of H. pylori but may take as long as 2 years. There is often a noticeable change in the histologic appearance of the biopsy within a few weeks with gradual clearance of the lymphoma in following months. Initially, there is disappearance of the inflammatory infiltrate accompanying the lymphoma with an empty-appearing eosinophilic lamina propria that may contain lymphoid aggregates (Fig. 18.20). These aggregates contain small B lymphocytes without transformed blasts and gradually become smaller with time. Immunohistochemistry shows that they contain few accompanying T cells and have a markedly reduced proliferation fraction compared to the original lymphoma. Such aggregates may not disappear altogether and may persist for long periods at the base of the mucosa or in the submucosa. In up to 60% of cases, B-cell monoclonality can still be demonstrated using PCR (50), suggesting that bacterial eradication represses but does not eliminate the lymphoma clone, which
is still presented in the lymphoid aggregates. The fate of these small aggregates is not completely known but it is assumed that they eventually disappear. PCR analysis may reveal persistence of the neoplastic clone after disappearance of morphologic evidence of lymphoma; however, the clinical significance of this finding is not clear. It is important not to make a diagnosis of persisting lymphoma on the basis of molecular analysis alone in the absence of good histologic evidence.
is still presented in the lymphoid aggregates. The fate of these small aggregates is not completely known but it is assumed that they eventually disappear. PCR analysis may reveal persistence of the neoplastic clone after disappearance of morphologic evidence of lymphoma; however, the clinical significance of this finding is not clear. It is important not to make a diagnosis of persisting lymphoma on the basis of molecular analysis alone in the absence of good histologic evidence.
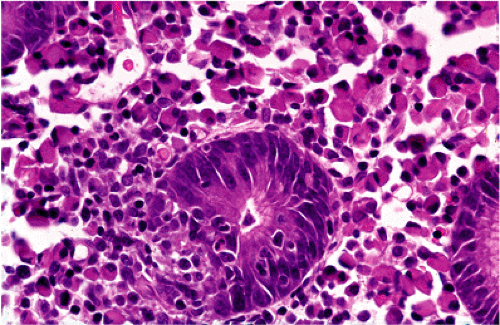 FIG. 18.12. Higher magnification of extreme plasma cell differentiation. A single lymphoepithelial lesion is present in the center. |
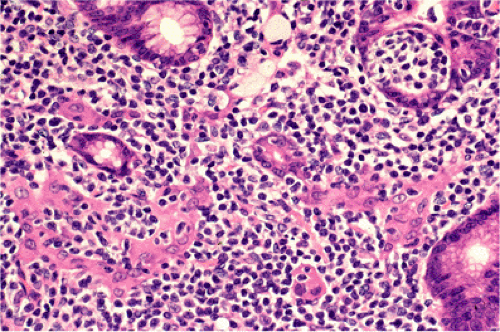 FIG. 18.13. Lymphoepithelial lesions in a case of gastric mucosa-associated lymphoid tissue lymphoma distorting glands and associated with eosinophilic change of gastric epithelium. |
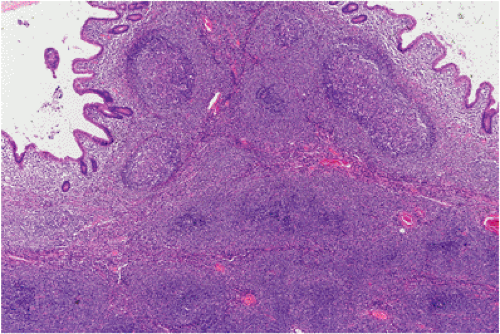 FIG. 18.14. Follicular colonization. Lymphoma cells in the marginal zone surround the follicles (above) and replace them (below), resulting in a follicular growth pattern. |
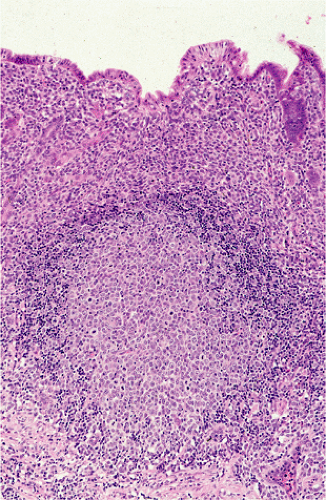 FIG. 18.15. The germinal center of a B-cell follicle has been infiltrated by transformed mucosa-associated lymphoid tissue lymphoma cells. |
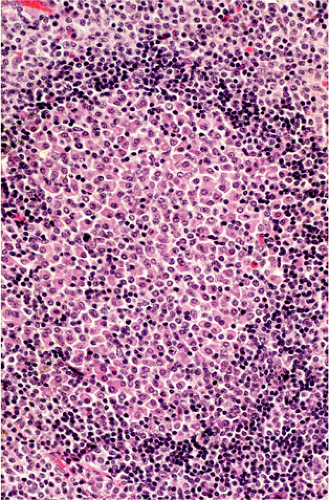 FIG. 18.16. In this case of gastric mucosa-associated lymphoid tissue lymphoma the germinal center is infiltrated by plasma cells. |
Multifocality of Gastric Mucosa-associated Lymphoid Tissue Lymphoma
Gastric MALT lymphoma typically disseminates within the stomach to form a multifocal lesion. Indirect evidence for this comes from the observation of recurrent MALT lymphomas in the gastric stump after partial gastrectomy in patients in whom clear resection margins were documented by histologic examination (51). Wotherspoon et al (52) systematically examined gastrectomy specimens of five MALT lymphomas using a “Swiss roll” technique. They showed that numerous small tumor foci with identical immunoglobulin light chain restriction to the main tumor mass were distributed throughout the gastric mucosa, including macroscopically normal regions (Figs. 18.21 and 18.22). A subsequent investigation by sequence analysis of the rearranged immunoglobulin heavy chain genes confirmed the clonal identity of these multiple tumor foci (53). Further studies by
microdissection and clone-specific PCR demonstrated that tumor cells were frequently present in reactive lymphoid tissue that showed no histologic evidence of lymphoma (54,55,56).
microdissection and clone-specific PCR demonstrated that tumor cells were frequently present in reactive lymphoid tissue that showed no histologic evidence of lymphoma (54,55,56).
Dissemination
Most gastric MALT lymphomas are at stage I when they present; between 4% and 17% have disseminated to regional lymph nodes, and approximately 10% have already disseminated to the bone marrow at the time of diagnosis (44,57). Gastric MALT lymphomas have a tendency to disseminate to other sites where MALT lymphomas occur, including the small intestine, salivary gland, and lung.
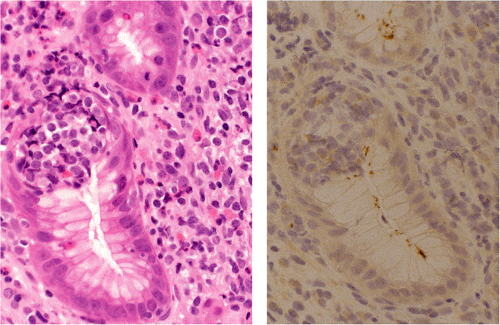
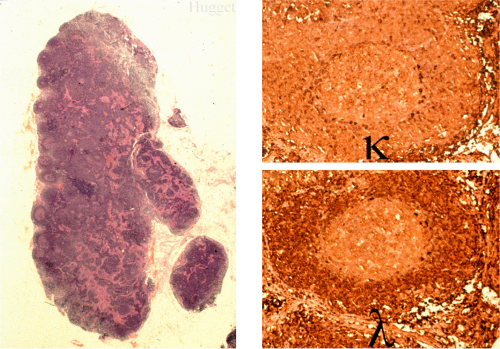
When MALT lymphomas disseminate to lymphoid tissue, including lymph nodes and spleen, they specifically invade the marginal zone (Fig. 18.23). This can lead to a deceptively benign or reactive appearance, especially in mesenteric lymph nodes, in which a marginal zone is normally present. Immunohistochemistry for immunoglobulin light chains can be very helpful in discriminating a normal marginal zone from disseminated MALT lymphoma (Fig. 18.24). Subsequently, the lymphoma in the marginal zones expands to form more obvious sheets of interfollicular lymphoma. Occasionally, follicular colonization in involved lymph nodes can lead to an appearance that simulates follicular lymphoma (Fig. 18.25).
Immunohistochemistry
The immunophenotype of MALT lymphoma essentially recapitulates that of marginal zone cells (Table 18.2). The B cells are CD20, CD79a, CD21, and CD35 positive and CD5, CD23, and CD10 negative. CD43, indicative of a neoplastic phenotype, is expressed in approximately 50% of cases. The tumor cells typically express IgM and less often IgA or IgG, are IgD negative, and show immunoglobulin light chain restriction (Fig. 18.26). A significant intratumoral population of CD3-positive, predominantly CD4-positive T cells is characteristic. Expanded meshworks of follicular dendritic cells are typically detected with antibodies CD21 and CD23, corresponding to follicles that have been overrun or specifically colonized by lymphoma cells. Variable numbers of CD10- and Bcl6-positive germinal center cells may be seen in
these areas, but the neoplastic MALT lymphoma cells are negative for these antigens.
these areas, but the neoplastic MALT lymphoma cells are negative for these antigens.
TABLE 18.2 Immunophenotype of Marginal Zone B Cells and Cells of MALT Lymphoma | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Molecular Genetic Features of Mucosa-associated Lymphoid Tissue Lymphoma
Antigen Receptor Genes
In the B cells of MALT lymphoma, immunoglobulin heavy and light chain genes are rearranged and show somatic mutation of their variable regions, consistent with a postgerminal center memory B-cell derivation (53,58). Ongoing mutations are thought to occur in most cases (58). Because of the difficulty in distinguishing between acquired MALT and MALT lymphoma, particularly in small biopsies (see below), there has been a tendency to rely on molecular evidence of monoclonality detected by PCR for the diagnosis of lymphoma. This technique may fail to detect monoclonality in up to 15% of cases of overt lymphoma and thus produce false-negative results (54). There are also reports of apparently spurious monoclonality in biopsies of acquired MALT, for example, gastric biopsies showing only chronic gastritis (42,55,56), where there is no histologic evidence of malignancy. The frequency of this spurious monoclonality varies between laboratories, which suggests that technique may be a factor. These findings serve to emphasize that MALT lymphoma should not be diagnosed in the absence of clear histologic evidence. This point is underlined by the frequent finding of persistent monoclonality in small residual, clinically insignificant lymphoid aggregates that persist following eradication of H. pylori for the treatment of MALT lymphoma (50).
Molecular Genetic Abnormalities
A number of molecular abnormalities have been described in gastric MALT lymphoma including trisomies 3, 12, and 18 and the specific chromosomal translocations t(11;18) (q21;q21), t(1;14)(p22;q32), and t(14;18)(q32;q21). T(11;18) involves the API2 and MALT1 genes and generates a functional API2–MALT1 fusion product (59,60,61). T(1;14) and t(14;18) juxtapose the BCL10 and MALT1 genes, respectively, to the immunoglobulin gene locus in 14q32 leading to deregulated expression of the oncogene (62,63,64,65). The oncogenic activities of the three chromosome translocations are linked by the physiologic role of BCL10 and MALT1 in antigen receptor-mediated nuclear factor (NF) κB activation
(66). The three chromosome translocations occur at markedly variable incidences in MALT lymphoma of different sites but are always mutually exclusive (66). Among the three chromosome translocations, t(11;18) is the most frequent, occurring in 25% to 30% of gastric MALT lymphomas (67).
(66). The three chromosome translocations occur at markedly variable incidences in MALT lymphoma of different sites but are always mutually exclusive (66). Among the three chromosome translocations, t(11;18) is the most frequent, occurring in 25% to 30% of gastric MALT lymphomas (67).
There is growing evidence suggesting that t(11;18)-positive cases are distinct from other MALT lymphomas, including those with t(1;14) or t(14;18). T(11;18)-positive MALT lymphomas rarely undergo high-grade transformation (68,69), despite the fact that the translocation is significantly associated with cases at advanced stages and that these cases typically do not respond to H. pylori eradication (70,71). Cytogenetically, t(11;18)-positive tumors usually do not show other chromosomal aberrations, such as trisomies 3 and 18, frequently seen in t(11;18)-negative tumors, including those positive for t(1;14) and t(14;18) (72). Furthermore, t(11;18) MALT lymphomas show markedly fewer chromosomal gains and losses than translocation-negative tumors (71).
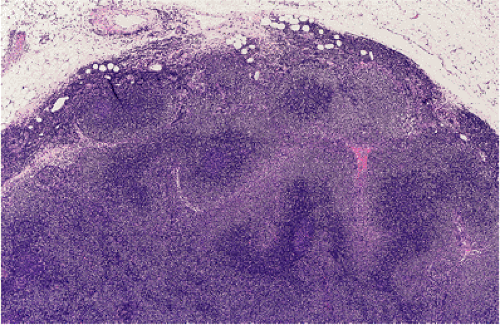 FIG. 18.23. Lymph node involvement by gastric mucosa-associated lymphoid tissue lymphoma. The tumor is distributed in the marginal zones. |
T(11;18) can be detected in paraffin-embedded tissue by reverse transcription PCR (RT-PCR), while fluorescent in situ hybridization (FISH) is useful for demonstrating all three characteristic translocations. In cases positive for t(11:18) as well as 20% of translocation-negative cases, BCL10 protein is up-regulated in the nucleus where it stains weakly (Fig. 18.27). In the much rarer t(1:14) cases, nuclear BCL10 is expressed intensely both in the nucleus and cytoplasm (Fig. 18.28). The significance of these findings remains unknown at present.
Postulated Normal Cell Counterpart of Mucosa-associated Lymphoid Tissue Lymphoma
The architectural features of MALT lymphoma, particularly in early cases, show quite clearly that the neoplastic cells are infiltrating the marginal zone around B-cell follicles (Figs. 18.7 and 18.29). In non-neoplastic lymphoid tissue a prominent marginal zone is present only in the spleen, Peyer patches,
and mesenteric lymph nodes. This allows a comparison of the cytology and immunophenotype of normal marginal zone cells with those of MALT lymphoma. Cytologically, MALT lymphoma cells bear a close resemblance to marginal zone cells. Both are slightly larger than small lymphocytes, have a slightly irregular nuclear outline, and moderate amounts of pale-staining cytoplasm. Interestingly, in Peyer patches collections of marginal zone cells are found within the dome epithelium. The immunophenotype of cells of the marginal zone and MALT lymphoma is virtually identical, both expressing CD20 and other pan B-cell antigens, CD21, CD35, and IgM, but not IgD.
and mesenteric lymph nodes. This allows a comparison of the cytology and immunophenotype of normal marginal zone cells with those of MALT lymphoma. Cytologically, MALT lymphoma cells bear a close resemblance to marginal zone cells. Both are slightly larger than small lymphocytes, have a slightly irregular nuclear outline, and moderate amounts of pale-staining cytoplasm. Interestingly, in Peyer patches collections of marginal zone cells are found within the dome epithelium. The immunophenotype of cells of the marginal zone and MALT lymphoma is virtually identical, both expressing CD20 and other pan B-cell antigens, CD21, CD35, and IgM, but not IgD.
Prognosis
MALT lymphomas are among the most indolent of all lymphomas and have a good prognosis overall regardless of stage. Five- and 10-year overall survival rates of over 80% are the rule, although progression-free survival may be somewhat lower (73). Cases in which transformation to diffuse large B-cell lymphoma has occurred have a significantly lower survival of approximately 50% at 5 years (48).
The treatment of gastric MALT lymphoma has attracted considerable attention since the initial publication showing that the lymphoma may regress following eradication of H. pylori with antibiotics. The follow-up of MALT lymphoma patients following eradication of H. pylori is rather complex, requiring repeated gastroscopy with biopsy (see above), and
it would be extremely useful to be able to identify the approximately 25% of cases of gastric MALT lymphoma that do not respond to eradication of H. pylori. Studies using endoscopic ultrasound have suggested that if the tumor has invaded beyond the submucosa, it is less likely to respond (74,75). Equally, cases that have transformed to large B-cell lymphoma are unlikely to respond, although there are reports of complete regression in such cases (76,77). Following the cloning of t(1;14) and t(11;18) breakpoints, these translocations have been shown to have a bearing on the response to H. pylori eradication. T(11;18)(q21;q21), present in up to 25% of cases, is strongly associated with failure to respond to eradication of H. pylori (78). Interestingly, both t(1;14) and t(11;18) are associated with nuclear expression of BCL10 protein that is particularly intense in t(1;14)-positive cases. Moreover, the frequency of both t(11;18)(q21;q21) and nuclear BCL10 expression is significantly higher in tumors that have invaded or disseminated beyond the stomach (78% and 93%, respectively) than those confined to the stomach (10% and 38%, respectively) (79). These findings in part explain the results based on the use of endoscopic ultrasound and suggest that both t(11;18)(q21;q21) and BCL10 nuclear expression are associated with failure to respond to H. pylori eradication and with more advanced stage MALT lymphoma. Therefore, before embarking on H. pylori eradication as definitive therapy, the pertinent genotypic and/or immunohistochemical investigations should be carried out.
it would be extremely useful to be able to identify the approximately 25% of cases of gastric MALT lymphoma that do not respond to eradication of H. pylori. Studies using endoscopic ultrasound have suggested that if the tumor has invaded beyond the submucosa, it is less likely to respond (74,75). Equally, cases that have transformed to large B-cell lymphoma are unlikely to respond, although there are reports of complete regression in such cases (76,77). Following the cloning of t(1;14) and t(11;18) breakpoints, these translocations have been shown to have a bearing on the response to H. pylori eradication. T(11;18)(q21;q21), present in up to 25% of cases, is strongly associated with failure to respond to eradication of H. pylori (78). Interestingly, both t(1;14) and t(11;18) are associated with nuclear expression of BCL10 protein that is particularly intense in t(1;14)-positive cases. Moreover, the frequency of both t(11;18)(q21;q21) and nuclear BCL10 expression is significantly higher in tumors that have invaded or disseminated beyond the stomach (78% and 93%, respectively) than those confined to the stomach (10% and 38%, respectively) (79). These findings in part explain the results based on the use of endoscopic ultrasound and suggest that both t(11;18)(q21;q21) and BCL10 nuclear expression are associated with failure to respond to H. pylori eradication and with more advanced stage MALT lymphoma. Therefore, before embarking on H. pylori eradication as definitive therapy, the pertinent genotypic and/or immunohistochemical investigations should be carried out.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree