Fig. 10.1
Diagram showing our “left lobe first choice” approach. Every donor is first screened for a left living donor hepatectomy following this algorithm
10.5 State-of-the-Art Preoperative Planning
Preoperative planning is arguably the most important factor involved in optimal outcomes for both donors and recipients. A thorough preoperative planning process integrates information gained from donor vascular, biliary, and volumetric analyses and recipient clinical and hemodynamic data to establish the best donor-recipient combination.
10.5.1 Donor Anatomical Evaluation
The first step in the assessment of a potential donor is a standard triple phase CT scan of the abdomen. A 1–2 mm incremental reconstruction is performed in both the arterial and venous phases to provide a very detailed arterial and venous anatomy of the donor as well as a volumetric analysis of the left/right lobes and FLR. Most centers obtain a preoperative assessment of the bile duct anatomy using magnetic resonance cholangiopancreatography (MRCP). CT cholangiography and ERCPs have been abandoned almost universally because the IV contrast agent used to opacify the biliary tree carries a relatively high risk of allergic reaction [16] and because of the potential for ERCP-related complications [17]. Additionally, all donors undergo an intraoperative cholangiogram to determine the anatomical and bile duct transection line.
If the CT scan and the MRCP show no contraindication for living donor hepatectomy, the images are elaborated to obtain a 3D rendering of the liver along with its vascular and biliary components (in our institution images are sent to MEVIS distant service; Bremen, Germany). 3D reconstruction (Fig. 10.2) carries several advantages over bi-dimensional imaging. First, it allows the representation of the portal vein, hepatic artery, and the bile duct bifurcation in one fused three-dimensional image that can be viewed, rotated, and manipulated to best predict and simulate the perspective encountered during surgery. Second, 3D reconstruction simulates the parenchymal transaction line with superimposed vascular and biliary structures, improving the surgeon’s ability to preoperatively understand the spatial relationships of these structures and therefore helps minimize intraoperative complications [18].
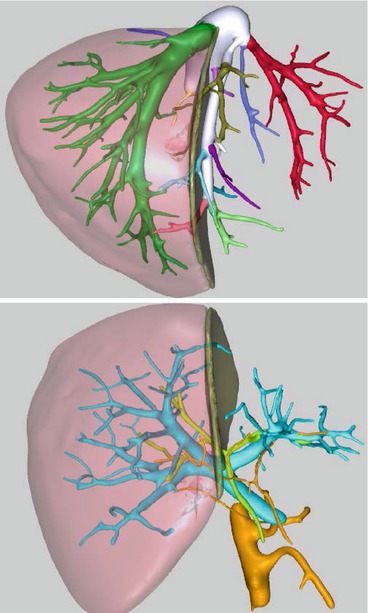

Fig. 10.2
MEVIS 3D reconstruction of the liver anatomy with proposed anatomical resection planes. Hepatic vein anatomy, combined portal vein, hepatic artery, and bile duct analysis
Additionally, 3D reconstruction provides an accurate assessment of the total and segmental liver volumes with a percentage of error of approximately 2.8 % [19, 20]. The estimated weight is obtained from volumes using a correction factor of 0.9 [21] and is used to calculate the estimated GRWR.
Finally, using a semi-automated process, 3D reconstruction depicts hepatic venous territories with the amount of parenchyma individually drained. This calculation is very important when making decisions about graft selection, inclusion/exclusion of the middle hepatic vein in the graft, and segmental venous reconstruction. These are all key functional aspects of partial graft transplantation, since suboptimal graft outflow is associated clearly with compromised regeneration, SFSS, and biliary complications [22, 23].
10.5.2 Innovations in Living Donor Preoperative Planning
10.5.2.1 3D Liver Printing
Three-dimensional (3D) printing is an innovative technology that produces solid objects starting from a digital image. Recently, our group was the first to describe [24] the use of this technology in LDLT. A total of six 3D-printed livers (three donors and three recipients) were analyzed prospectively and were found to be extremely accurate in depicting the spatial relationships and dimensions of the vascular and biliary structures of the actual livers (Fig. 10.3). 3D printing, though in its infancy, offers several theoretical advantages over the current 3D screen-only graft reconstruction. Importantly, having a physical model of the liver gives the surgeon a tool for intuitive navigation of the critical anatomical structures found during surgery due to the transparency of the model and the use of color “ink” to print vascular and biliary structures. Future refinement of 3D printing and the modeling process may complement current imaging technology and provide surgeons with an additional planning tool.
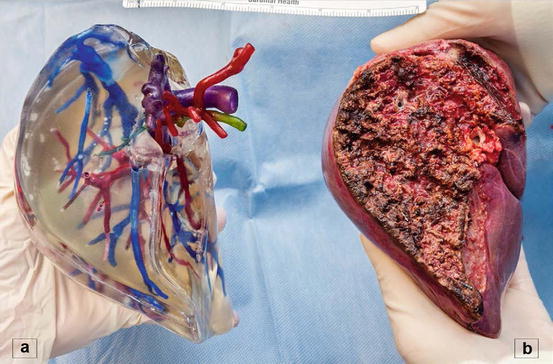

Fig. 10.3
3D printed right hemiliver (a) with the respective graft (b)
10.5.2.2 Computer-Assisted Surgical Navigation
Computer-assisted surgery is used in numerous fields and only recently has been introduced in liver surgery. This technology involves the use of a tracking device and a computer system that integrates, in real time, the field position of the surgical instrument with the preoperative imaging of the patient. The tracked instrument position is overlaid on multiple CT/MRI images, improving the surgeon’s spatial understanding of the vital structures that will be encountered during parenchymal dissection, thereby helping to minimize complications. While tracked surgical devices hold great potential in LDLT, the lack of accuracy during mobilization and repositioning of the liver is a current and significant limitation.
10.5.3 Recipient Evaluation
In order to optimize donor and recipient outcomes, preoperative donor assessments need to be integrated with recipient imaging and clinical information.
The recipient’s clinical characteristics, such as MELD score, severity of portal hypertension, and hemodynamic parameters (CO, CI), are combined with imaging data, including spleen dimension and the presence of portosystemic shunts to guide the choice of the graft. High-risk patients, such as those with a high MELD score or severe portal hypertension, should be considered for LDLT only if a GRWR > 0.8 can be safely obtained. Conversely, recipients with large portosystemic shunts and therefore decompressed portal venous system can be considered for smaller grafts, ideally left lobe.
10.6 Surgical Techniques
10.6.1 Graft Type and Surgical Planning
10.6.1.1 Donor Surgery
Unlike liver resection for malignancy, both portions of the graft and the remnant liver must be handled with extreme caution in donor hepatectomy. Preservation of vascular and biliary integrity on both sides of the liver is critical. Donor safety depends on preoperative knowledge of surgical anatomy, which is highly variable. The presence of multiple variants may be a relative contraindication for donation, particularly in high-risk cases.
Several important principles must be followed in donor surgery to maximize donor safety. First, contralateral hepatic ligaments should not be divided. The division of these ligaments can cause malrotation of the remnant liver, which in turn may cause catastrophic vascular insufficiency in the donor. Second, an intraoperative cholangiogram should be performed before hilar dissection to rule out anatomical variants that are not found preoperatively. Third, hepatic hilar dissection on the side of the hepatic lobe or segment should be minimized to avoid compromising blood supply to the bile duct. Fourth, a confirmatory cholangiogram should be performed after the graft liver is removed to confirm the integrity of the biliary system. Fifth, after a right hepatectomy, the falciform ligament should be tagged to the anterior abdominal wall to avoid malrotation of the remnant liver. In addition to these, the use of the liver hanging maneuver helps minimize blood loss and guides the direction of parenchymal transection [25]. Liver parenchymal division should be performed with familiar methods (CUSA, clamp-crushing technique, water-jet, bipolar coagulator, etc.) that further help minimize blood loss.
While experienced centers have performed donor hepatectomy through minimally invasive techniques, general application of this approach remains controversial due to concerns about the difficulty of controlling inadvertent massive bleeding under a laparoscopic view. Furthermore, surgical stress on living donors seems to be determined by the amount of liver mass removed during donor surgery rather than the size of incisions. Therefore, further studies and extensive discussion will be necessary before reaching a general consensus on minimally invasive donor hepatectomy.
The extent of venous reconstruction varies considerably between the left and the right lobe. The left lobe graft usually needs a simple venoplasty to optimize venous outflow on the back table [26]. In contrast, the right lobe graft frequently needs complex venous reconstruction. Reconstruction of segment five and eight veins increases the functional liver mass so that it is comparable to the extended right lobe graft with the middle hepatic vein. Vascular grafts for this reconstruction can be taken from unused vessels procured from deceased donors, cryopreserved grafts, and autologous veins such as recipient intrahepatic portal vein, internal jugular vein, and recanalized umbilical vein [27]. If these grafts are not available, expanded polytetrafluoroethylene grafts can be used with excellent early patency [28]. When two separate hepatic ducts are identified, these can be combined together using a ductoplasty technique to avoid multiple biliary reconstructions in the recipient.
10.6.1.2 Recipient Surgery
Two major components of recipient surgery are essential to achieve good outcomes: excellent venous outflow to prevent graft congestion and active portal inflow modification to optimize graft hemodynamics. In the small pediatric cases, venous outflow can be maximized by making a vertical cavotomy on the retrohepatic cava from the common orifice of the three hepatic veins to create a triangular shape orifice [29]. This technique was first described in 1988, but remains the gold standard of outflow venous reconstruction in pediatric partial grafting. This technique can also be applied for the left lobe graft in adults. If the recipient venous orifice is too big and does not match the donor hepatic vein, the alternative technique for left lobe venous reconstruction is to make a horizontal cavotomy on the right side of the middle hepatic vein to enlarge the size of the common channel of the left and middle hepatic veins. In the right lobe graft, multiple venous anastomoses are frequently required, including the right hepatic veins and anterior segment branches. When a venous patch is available, multiple venous orifices can be combined together to perform one-step venous anastomosis [30]. Suboptimal venous outflow decreases functional graft size and increases the risk of graft dysfunction or failure.
In size-mismatch adult LDLT, a small graft receives excessive portal flow causing an arterial spasm via hepatic arterial buffer response, which explains the pathophysiology of SFSS [31]. Splenic artery ligation is the most frequently used technique for inflow modulation, but the effect of portal flow reduction is not always promising. Splenectomy is more effective but less frequently used due to the risk of increased blood loss and post-splenectomy sepsis [32]. Portosystemic shunt continues to be used by some centers with excellent outcomes, but there is an increased risk of portal flow steal resulting in graft hypoperfusion [33].
10.7 Recipient Outcomes
In pediatric patients, graft and patient survival is comparable or better for LDLT than deceased donor liver transplantation. This result is consistent with the historical observation in split liver transplantation using deceased donors. In LDLT for adults, national data in the USA has shown that the outcomes in LDLT are comparable to deceased donor liver transplantation in terms of survival, cost, and hospital mortality, despite the increased risk of early complications [34]. Moreover, an intent-to-treat analysis has proved a significant survival benefit of recipients who received livers from living donors compared to deceased donors [35, 36].
Interestingly, the outcome advantage for LDLT is sustained even for those recipients with a MELD score of <15 [37], a group that generally does not benefit from DDLT.
10.7.1 Hepatocellular Carcinoma
Early studies raised the concern that the high regeneration rate of LDLT grafts early after transplant could induce HCC and HCV recurrence. A single-center study showed higher tumor recurrence rate after LDLT compared to that of DDLT [38]. Recently, a multicenter study by the A2ALL group confirmed the finding [39]. The study suggested that the higher recurrence rate was due to advanced staged tumors in the LDLT group. More specifically, the shorter waiting period between diagnosis or locoregional therapy and LDLT seemed to allow recipients with aggressive HCC to undergo LDLT. The longer waiting time for DDLT seemed to result in a pool of transplant recipients who had less aggressive tumors. Interestingly, the study suggested a mandated observation time after locoregional therapy before LDLT for recipients with advanced HCC in order to determine tumor biological behavior and to exclude candidates with aggressive HCC who likely would not benefit from LDLT. The study suggests that such an approach would result in comparable outcomes following both LDLT and DDLT for HCC.
10.7.2 Hepatitis C
HCV cirrhosis is the important indication for both DDLT and LDLT. While early data suggested that HCV may recur earlier and the incidence of severe recurrence is higher for HCV patients undergoing LDLT [40, 41], recent studies demonstrate that the outcome is approximately equal to that of DDLT [42].
10.8 Complications
10.8.1 Donor Complications
To date, more than 11,500 LDLTs have been performed worldwide with a total of 34 living liver donor deaths reported in the literature [43]. A longitudinal observational cohort of 740 living donor hepatectomies (707 right/33 left hepatectomies) performed at nine major US centers (A2ALL consortium) [43] experienced an overall complications rate of 40 %, with a 1 % incidence of catastrophic complications (residual disability, liver failure, or death) and 2–5 % risk of aborted donation. These complication rates were confirmed by other groups [44, 45]. Infections represented the most common complication (10 %). Bile leak/biloma accounted for 8 % of complications, followed by incisional hernia 6 %, psychological complications 6 %, neuropraxia 3 %, ileus 3 %, unplanned re-exploration 2 %, ascites 2 %, pleural effusion 1.8 %, bowel obstruction 1.6 %, DVT/pulmonary embolism 1.5 %, intra-abdominal abscess 1 %, intra-abdominal bleeding 0.9 %, and biliary stricture 0.6 %. Based on the existing literature, the risk of donor death after a right hepatectomy is 0.4–0.6 % and for a left-sided hepatectomy is 0.1–0.4 %.
10.8.2 Recipient Complications
Given its technical complexity, it is not surprising that LDLT carries higher complication rates compared to DDLT. Biliary complications remain the Achilles heel of LDLT, and most of the technical complications occur in the first 90 days after transplantation.
In a report of 385 transplants performed in the USA [46], early bile leaks were seen in 30 % of recipients, and biliary strictures were observed in 8 %. Vascular complications occurred in 8 % of patients (HAT 6 %, PV thrombosis 2 %). Intra-abdominal bleeding presented in 7 % of the patients, and a re-exploration was needed in 24 % of the cases. As center experience increased, the rate of biliary complications decreased from 38 % (first 20 cases) to 24 %. A similar trend was found in the rate of hepatic artery thrombosis 8–4 % and PV thrombosis (3–1 %). Biliary stricture (10 %), infections (8 %), and hernias (5 %) represented the most common complications after 90 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






