Fig. 11.1
Three examples of frozen-section analysis on donor liver biopsies with 0 % (a), 10 % (b) and >30 % (c) steatosis. In (b, c) freezing artifacts are visible as empty spaces, representing a pitfall in the evaluation of macrosteatosis. Magnification 10×
11.2 Graft Dysfunction
11.2.1 Preservation Injury
Also called “ischaemia/reperfusion injury”, preservation injury is the leading cause of early graft dysfunction and is defined as tissue damage occurring immediately after graft reperfusion, in the absence of other explicable causes of liver injury (e.g. vascular or biliary; see below).
The histopathological features of preservation injury depend on when the damage takes place: a warm ischaemia time of 120 min or more compromises both hepatocytes and endothelial cells, while a prolonged (usually more than 12 h) cold ischaemia is characterized by sinusoidal and endothelial damage, with a higher incidence of biliary complications [5, 6]. The reperfusion phase is the most crucial for the onset of liver preservation damage, since the reoxygenation after ischaemia causes activation of the Kupffer cells and complement factors, resulting in the production of reactive oxygen species and cytokines. The short-term consequences are granulocyte migration in the sinusoids and general vasoconstriction that can lead to graft circulatory failure [7].
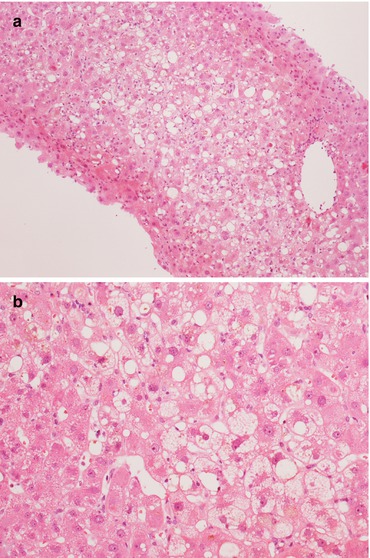
Fig. 11.2
(a, b) Ischaemic lobular damage, characterized by hepatocyte swelling and ballooning, without a significant inflammatory infiltrate. Magnification 10× (a); 20× (b)
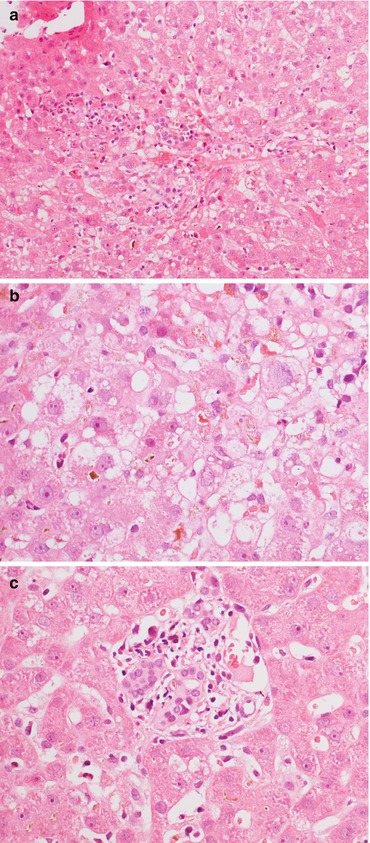
Fig. 11.3
(a) In some cases of ischaemia/reperfusion injury, architectural distortion, lobular haemorrhage with hepatocyte polymorphism and ballooning are visible. (b) Cholestasis and histiocytosis in zone 3 can be seen several weeks after OLT, associated with (c) portal tract distortion and bile duct regression. Magnification 20× (a); 40× (b, c)
Microscopically, mild preservation injuries are visible on biopsies taken 1 h after OLT as microvesicular steatosis with hepatocyte swelling, but these features are rapidly reversible. Conversely, the more severe forms of preservation injury are characterized microscopically by hepatocyte necrosis, especially in zone 3, with acidophilic bodies and/or zonal or confluent necrosis. The adjacent viable hepatocytes can show ballooning and mitotic activity. Bile duct degeneration is visible as a detachment of the biliocytes from the basement membrane and as a ductular reaction with ductular and lobular neutrophilic infiltrate [8, 9]. As a consequence, both intracellular and extracellular cholestases (with bile plugs) are common. Cholestasis, architectural lobular distortion, hepatocyte mitotic activity with nuclear polymorphism and ballooning, as well as histiocytosis in zone 3 can be seen several weeks after OLT.
Differential Diagnosis
In these early phases, the differential diagnosis includes acute infections, biliary complications, and antibody-mediated rejection. Cholangitis, bile duct obstruction, and reperfusion injury share predominant zone 3 damage, but cholangitis is commonly characterized by periductal oedema with neutrophilic granulocytes [9]. Interestingly, immunohistochemical positivity for C4d has been demonstrated in necrosis during reperfusion injury [10].
11.2.2 Hyperperfusion (“Small-for-Size” Syndrome)
This is a very early post-transplant complication (within 2 weeks), due to inadequate graft size (less than 0.8 % of the recipient’s weight) resulting in hyperdynamic portal flow. Allograft hyperperfusion is a critical condition that generally requires retransplantation.
Histopathology
The histopathological modifications of “small-for-size” syndrome are commonly sorted into early, intermediate and late changes [11]. Starting from a few minutes after reperfusion, denudation of portal veins and sinusoids can be seen, resulting in haemorrhages in the periportal zone (zone 1), all consequences of portal hyperperfusion. In the more severe cases, ischaemic bile duct damage with lobular infarcts can be seen [9]. Intermediate features include hypertrophic changes in the endothelia, oedema and fibrosis, while the late changes comprise thrombosis and obliteration of the portal veins with recanalization, together with nodular regenerative hyperplasia and biliary stenosis.
Differential Diagnosis
Arterial vascular complications (see below).
11.2.3 Vascular Complications
Post-OLT vascular complications include hepatic artery thrombosis, portal vein thrombosis and hepatic venous outflow obstruction (HVOO). Vascular complications represent a major technical problem after OLT and an important cause of liver damage and dysfunction in the first months after transplantation. Since liver biopsy is neither reliable nor safe in this setting, and the affected vascular structures are hilar and perihilar, histopathological examination is generally confined to the graft explant specimen [9].
Histopathology
At gross examination, the liver surface may be normal, while foci of cholestasis and/or necrosis can be seen in some cases. At histology, thrombosis of the hepatic artery leads to ischaemic necrosis of the bile ducts with epithelial denudation and bile leakage into the surrounding parenchyma. Lobular alterations, ranging from Councilman’s bodies to confluent hepatocytic necrosis, appear in the more severe and advanced cases. Prolonged arterial ischaemic injury can result in biliary strictures and fibrosis [12].
Histopathological examination of an allograft with portal vein thrombosis shows various degrees of parenchymal damage, from hepatocyte swelling and necrosis with haemorrhage and eventually thrombosis in the areas around the portal branches, to a pan-lobular necrosis of the allograft in cases with massive portal thrombosis. Chronic portal obstruction leads to nodular regenerative hyperplasia or similar changes [9].
HVOO can be due to problems at different levels, from congestive heart failure to Budd-Chiari syndrome and veno-occlusive disease affecting the portal venules or even sinusoids. The clinical presentations and the aetiologies of these conditions vary widely, but the histopathological picture is common. The first alterations affect the centrolobular zone (zone 3), with sinusoidal dilatation and centrolobular haemorrhage (in advanced cases), hepatocyte atrophy and loss of lobular architecture. Lobular or portal inflammation is lacking, and only the most severe cases show cholestasis. Chronicization (e.g. in Budd-Chiari syndrome, which is often subacute) leads to bridging fibrosis and the formation of hepatocellular nodules resembling focal nodular hyperplasia [12].
11.2.4 Large Bile Duct Obstruction
Strictures of the large bile ducts can be due to technical complications involving anastomoses, or other causes, including severe ischaemic and preservation injuries (see above) and chronic rejection with arteriopathy, among others.
Histopathology (Fig. 11.4)
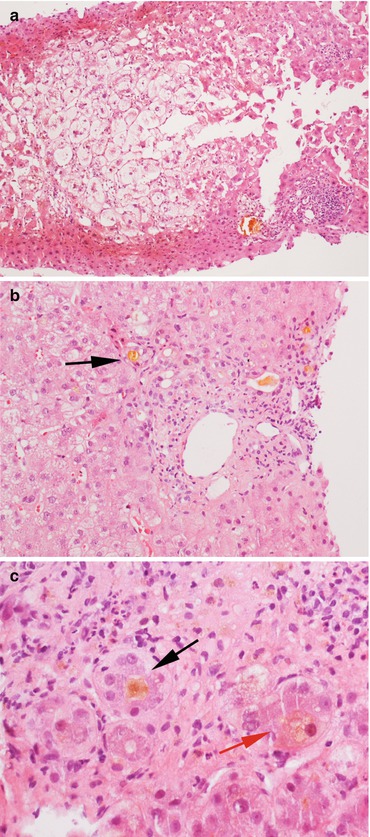
Fig. 11.4
A severe case of post-transplant biliary obstruction. (a) The lobule shows marked feathery degeneration of the hepatocytes and architectural distortion. (b, c) The portal tracts show inflammation with neutrophilic granulocytes, neoductulogenesis with bile plugs (black arrows) and intracellular cholestasis (red arrow). Magnification 10× (a); 20× (b); 40× (c)
Post-transplant biliary complications histologically resemble the biliary disease of the native liver. Portal oedema and inflammation with neutrophilic granulocytes, resembling cholangitis, are common findings: the predominance of the neutrophilic infiltrate is very important in the differential diagnosis between post-OLT biliary complications and acute rejection. Cholestasis can be associated, while portal fibrosis and biliary cirrhosis represent the chronic evolution of this condition [12, 13].
11.3 Rejection
11.3.1 Acute Cellular Rejection
Acute cellular rejection (ACR) is defined as a predominantly lymphocytic inflammation of the graft due to an antigen mismatch between donor and recipient. The liver structures most commonly affected are the bile ducts and the vascular endothelia. Due to the high variability in onset and clinical symptoms, liver biopsy is mandatory in cases of suspect ACR.
Histopathology (Fig. 11.5)
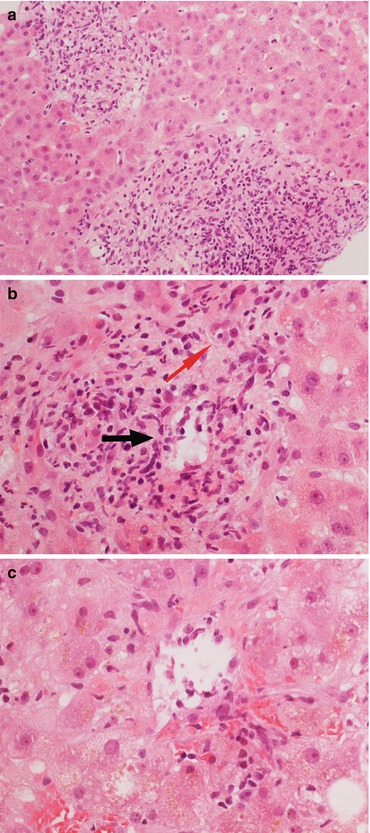
Fig. 11.5
(a) Acute cellular rejection (ACR) is characterized by a portal inflammatory infiltrate with portal expansion. (b) The inflammatory infiltrate is prevalently lymphocytic, although macrophages and granulocytes can be present. There is aggression of the vascular (black arrow) and biliary (red arrow) structures. (c) Endothelialitis is defined as the presence of inflammatory cells in the subendothelial layer of the graft vascular structures (in this case, a centrolobular vein). Magnification 20× (a); 40× (b, c)
The main histological features of ACR are portal inflammatory infiltrate, endothelialitis and bile duct aggression. The inflammatory infiltrate characterizing ACR is mainly portal and with a prevalent lymphocytic component, although macrophages and both neutrophilic and eosinophilic granulocytes can be seen. The lymphocytes are CD8-positive and show the morphology of activated (or frankly blastic) T cells [14]. Lymphocyte-mediated bile duct injury is always present, with intraepithelial lymphocytes and associated bile duct regressive and reactive changes such as cytoplasmic eosinophilia and/or vacuolation, prominent nucleoli and occasional apoptosis [15]. Endothelialitis is defined as the presence of inflammatory cells in the subendothelial layer of the graft vascular (mainly venous) structures [16]. It is an important diagnostic feature in ACR, albeit not a specific finding since it can be detected in other pathological conditions (e.g. HCV recurrent hepatitis, infections) [17].
Differential Diagnosis
Recurrent and de novo viral hepatitis (HCV, HBV, CMV, EBV), drug hepatitis, lymphoproliferative disorders and other disorders with a predominant inflammatory component. Clinical onset, serological and clinical data are mandatory for the differential diagnosis. The presence of a plasma cell component requires the differential diagnosis from plasmacellular HCV recurrent hepatitis or post-OLT autoimmune hepatitis: the role of plasma cells in acute rejection is still debated [18] (Fig. 11.6).
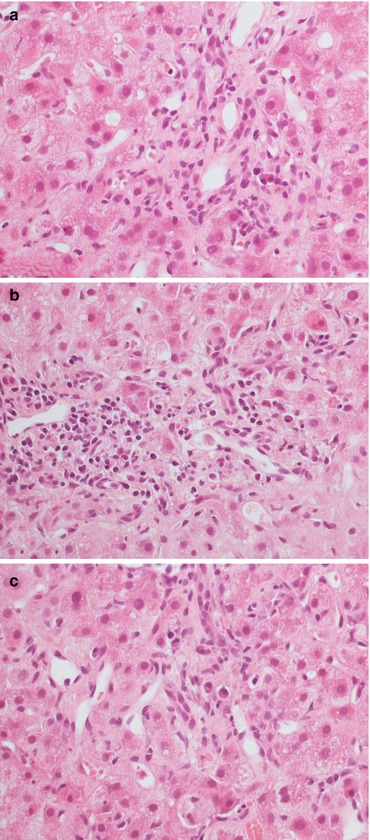

Fig. 11.6
A scarce (a) or more pronounced (b) plasma cell component, both in the portal tracts (a, b) and lobule (c) can be associated with rejection, recurrent viral hepatitis and de novo autoimmune hepatitis. The meaning of the plasma cell infiltrate after transplantation is controversial. Magnification 40×
Histopathological ACR is graded according to the 1997 Banff criteria as follows [14]:
Histopathological parameter | Description | Rejection activity index |
|---|---|---|
Portal inflammation | Mild/focal | 1 |
Diffuse, mixed with occasional blasts and granulocytes | 2 | |
Diffuse, mixed with numerous blasts and granulocytes, and interface activity | 3 | |
Bile duct involvement | Mild/focal inflammation | 1 |
Diffuse inflammation with epithelial injury to a few ducts | 2 | |
Diffuse inflammation of most bile ducts; biliary cells necrosis | 3 | |
Endothelialitis | Presence of subendothelial lymphocytes in a minority of portal vessels | 1 |
Presence of subendothelial lymphocytes in most portal and centrolobular vessels | 2 |
The absence of one or more criteria might lead to a diagnosis of indeterminate for rejection (Figs. 11.7 and 11.8), while according to these criteria a rejection activity index (RAI) of three to four represents mild ACR, a RAI of five to six moderate ACR, and a RAI greater than six severe ACR [14].
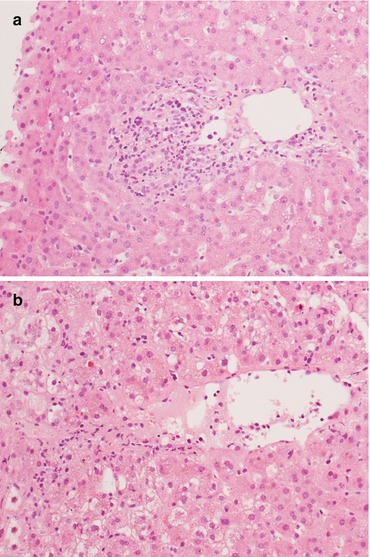
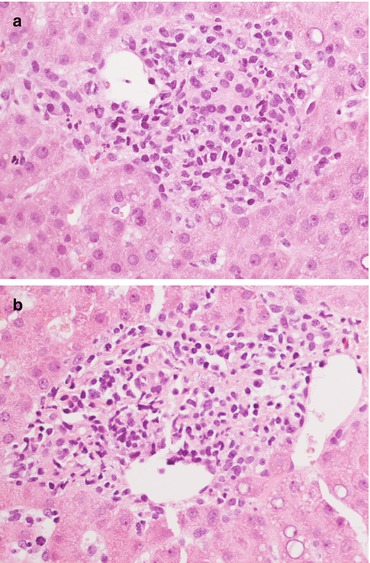

Fig. 11.7
A histological picture with only one or two criteria for rejection poses the diagnosis of indeterminate for rejection according to Banff. In these cases there can be (a) a mild portal infiltrate possibly with bile duct aggression or (b) mild and focal endothelial aggression of centrolobular veins. Magnification 20×

Fig. 11.8




(a, b) Detail on portal tract involvement in indeterminate for rejection, characterized by an inflammatory infiltrate, marked bile duct regression and focal subendothelial lymphocytes. Magnification 40×
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






