Benign
Hemangioma: focal, multiple, diffuse
Mesenchymal hamartoma
Hepatic adenoma
Focal nodular hyperplasia
Inflammatory myofibroblastic tumor (may be locally invasive)
Malignant
Primary
Hepatoblastoma
Hepatocellular carcinoma, fibrolamellar HCC
Transitional tumors
Embryonal sarcoma
Biliary rhabdomyosarcoma
Calcifying nested stromal–epithelial tumor
Angiosarcoma
Secondary
Lymphomas, leukemia, Wilms’ tumor, neuroblastoma, osteosarcoma, colon cancer
Table 69.2
Risk factors and premalignant conditions for childhood liver tumors
Tumor | Risk factors | Premalignant lesions |
|---|---|---|
Hepatoblastoma | Beckwith–Wiedemann syndrome,familial adenomatous polyposis, Li–Fraumeni syndrome, trisomy 18, preterm birth and very low birth weight | – |
Hepatocellular carcinoma | Glycogen storage disease, tyrosinemia, Alagille syndrome, biliary atresia, PFIC, Ataxia-telangiectasia, hepatitis B infection, hepatitis C infection | Hepatic adenoma |
Embryonal sarcoma | – | Mesenchymal hamartoma |
Angiosarcoma | – | Hemangioma |
There is a striking age-related variation in the frequency of different tumor types (Table 69.3). Over 90 % of liver tumors in children below 5 years are HB, while 87 % of tumors in the 15–19-year age group are HCC. A gradual increase in the incidence of liver tumors in children over the past 3–4 decades has been reported. This is particularly evident in the case of HB where the incidence has increased from 0.6 to 1.2 per million population between 1973–1977 and 1993–1997. On the contrary, incidence of HCC has decreased from 0.45 to 0.29 per million population during the same period [2].
Table 69.3
Age-wise distribution of childhood liver tumors
Age group | Benign tumors | Malignant tumors |
|---|---|---|
Neonatal period | Hemangioma, mesenchymal hamartoma | Hepatoblastoma |
0–5 years | – | Hepatoblastoma, biliary rhabdomyosarcoma |
5–15 years | – | Hepatocellular carcinoma, embryonal sarcoma |
> 15 years | Hepatic adenoma | Fibrolamellar carcinoma |
Tumor Markers in Childhood Liver Tumors
Alpha-fetoprotein (AFP) is the most recognized tumor marker in liver tumors . AFP is a glycoprotein similar in physical and chemical characteristics to albumin. It is secreted by the fetal liver and yolk sac until 13 weeks’ gestation and then primarily by the fetal liver [3]. AFP levels at birth are very high with Bader et al. reporting a median level of over 40,000 ng/ml in cord blood samples [4]. These levels rapidly drop during the first year of life at a rate primarily dictated by the half-life of AFP of 5–6 days [5]. AFP levels at birth in the preterm babies are higher than full-term babies. Similarly, a decrease in at-birth AFP for every week of prolonged gestation has been reported [4]. The high levels of AFP in the infant should be kept in mind by the clinician when AFP levels are used for the diagnosis and monitoring of pediatric liver tumors .
Several tumors are associated with elevated AFP. HB is the commonest cause in infants though HCCs and germ cell tumors are also associated with elevated AFP. Nonneoplastic conditions, such as tyrosinemia and neonatal hepatitis, can also cause elevated AFP levels.
Malignant Tumors
Hepatoblastoma
HB is the most common liver tumor in children. It is almost always seen in the first 4 years of life with median age at diagnosis of 18 months. It can present at birth and has been diagnosed in the intrauterine period on antenatal scans. Prematurity and very small birth weight have been identified as risk factors. HB developing in these children is also reported to have a worse prognosis [6, 7].
HB is a tumor of immature hepatocyte progenitor cells. It is an embryonal tumor, which recapitulates various stages of liver development. Histologically, these tumors are heterogeneous and comprise combinations of epithelial, mesenchymal, and occasionally teratoid components in varying proportions (Table 69.4, Fig. 69.1). Majority of tumors have a primarily epithelial component containing hepatoblasts at varying stages of differentiation. The histological type has an impact on behavior with well-differentiated fetal epithelial type having the best prognosis. The small-cell undifferentiated type of HB has the worst prognosis with very poor survival. Mixed tumors contain both epithelial and mesenchymal components, are more resistant to chemotherapy, and have a worse prognosis. Post-chemotherapy residual tumors and metastatic tumors may demonstrate a pleomorphic pattern with pleomorphic nuclei having coarse chromatin and prominent nucleoli. This pattern may resemble HCC .
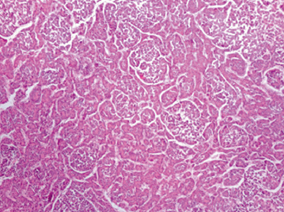

Fig. 69.1
Hepatoblastoma, epithelial type with fetal and embryonal epithelium.
Table 69.4
Histological types of hepatoblastoma
Histological type | Description |
|---|---|
Epithelial | Primarily containing immature hepatocytes |
Fetal | Commonest variant of epithelial HB. Composed of polygonal cells resembling fetal hepatocytes arranged in one- to two-cell thick cords, trabeculae or sheets |
Embryonal, well differentiated | Tumor resembles the liver at 6–8 weeks of gestation. Demonstrates organized tubular or acinar formation. Hematopoietic elements are commonly admixed with epithelial component |
Cholangioblastic | Tumour cells differentiate as cholangiocytes and form small ducts. This component expresses cholangiocyte lineage markers (cytokeratins 7 and 19) |
Macrotrabecular | Cells arranged in thick trabecular pattern (> 5 cells thick) |
Small cell undifferentiated | Sheets of small cells with large hyperchromatic nuclei similar to neuroblastoma |
Mixed epithelial–mesenchymal type | Mixture of epithelial and mesenchymal cell types |
Teratoid | Contains heterologous components such as stratified squamous epithelium, mucus-producing cells, neuroectodermal derivatives |
Non-teratoid | Contains stromal derivatives including spindle fibroblastic cells, osteoid, skeletal muscle, and cartilage |
Diagnosis
The usual presentation is with an abdominal lump identified by the parent or the clinician. Pain, failure to thrive, or jaundice are uncommon modes of presentation. Investigations reveal an elevated AFP level in over 90 % of cases. The AFP levels are extremely high (in the order of 105 ng/ml) and are usually a log higher than those seen with HCC. AFP level has been identified as a prognostic marker in HB with both very high levels and low levels (< 100 ng/ml) predicting poor biology [8]. Thrombocytosis is a recognized laboratory finding in HB and is probably related to production of thrombopoietic cytokines including thrombopoietin in the tumor tissues.
Imaging with computed tomography (CT) is necessary to confirm the diagnosis, stage the extent of liver disease, identify vascular invasion, extrahepatic disease, and lung metastases. HB appears as a heterogeneously enhancing well-circumscribed lesion with occasional calcifications. A biopsy is usually required to confirm diagnosis before start of neoadjuvant chemotherapy and for prognostication.
Staging and Prognostication
Several staging systems have been developed to tailor treatment for HB. The preoperative extent of tumor (PRETEXT) system based on preoperative imaging, which was developed and popularized by the International Childhood Liver Tumors Strategy (SIOPEL) Group, is commonly used in Europe (Table 69.5, Fig. 69.2). This is an anatomical classification focusing on the extent of tumor and the amount of liver that can be spared during resection [9]. Modifications to the PRETEXT scoring system have included additional subclassifications to identify high-risk factors such as vascular involvement, caudate lobe involvement, etc. (Table 69.6) [10].
SIOPEL also classifies HB into standard risk, high risk, and very-high-risk groups based on the PRETEXT staging and additional factors to tailor management (Table 69.7) [11]. Other staging systems include the Children’s Oncology Group (COG) classification, which is based on intraoperative findings, and presence of residual tumor have been used more commonly in the USA.
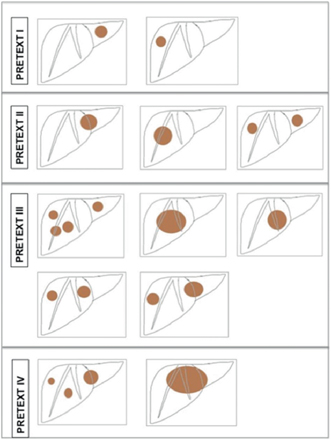

Fig. 69.2
PRETEXT staging: The PRETEXT system is based on the preoperative imaging and is an assessment of the liver that is free of tumor. The liver is divided into four sectors by the right and middle hepatic veins and the falciform ligament. The four sectors are the left lateral, left medial, right medial, and right lateral sectors. The number of contiguous sectors that are free of tumor is the key to the staging. Tumor may be single or multiple. a PRETEXT 1, three contiguous sectors are tumor free, b PRETEXT 2, two contiguous sectors are free of disease, c PRETEXT 3, only one sector is free, d PRETEXT 4, all four sectors are involved
Table 69.5
PRETEXT staging and frequency of various PRETEXT stages at initial presentation [8]. Refer to Fig. 69.2
PRETEXT staging | Definition | Frequency at presentation (%) |
|---|---|---|
I | One section is involved and three adjoining sections are free | 4.8 |
II | One or two sections are involved, but two adjoining sections are free | 36.6 |
III | Two or three sections are involved and no two adjoining sections are free | 38.8 |
IV | All four sections are involved | 19.8 |
Caudate lobe involvement | C1—tumor involving caudate lobe | – |
C0—all other patients | ||
Extrahepatic abdominal disease | E0—no evidence of tumor spread in abdomen (except M or N) | Add suffix “a” if ascites is present |
E1—direct extension of tumor into adjacent organs or diaphragm | ||
E2—peritoneal nodules | ||
Tumor focality | F0—solitary tumor | – |
F1—two or more discrete tumors | ||
Tumor rupture | H1 | – |
Distant metastases | M1 | – |
Lymph node metastasis | N0—no nodal metastases | – |
N1—abdominal lymph node metastases only | ||
N2—extra-abdominal lymph node metastases | ||
Portal vein involvement | P1—involvement of left or right branch of portal vein | Add suffix “a” if intravascular tumor present |
P2—involvement of main portal vein | ||
IVC or hepatic vein involvement | V1—involvement of one hepatic vein, IVC free | Add suffix “a” if intravascular tumor present |
V2—involvement of two hepatic veins, IVC free | ||
V3—involvement of all three hepatic veins and/or IVC |
Management
Early results of HB with surgical resection alone were poor due to the advanced stage at which these tumors usually present. Only 5 % of all tumors at presentation can be staged as PRETEXT I, and over 50 % are unresectable at initial presentation (Table 69.5). Metastatic disease in the lungs at presentation is not uncommon. However, this should not dissuade the clinician from aiming for cure as resection of lung metastases along with treatment of primary has been shown to improve long-term survival [12].
HB are highly chemosensitive tumors and respond well to platinum-compound-based chemotherapy. Use of chemotherapy in conjunction with surgery has radically altered the outcomes of these tumors. Today, multimodality treatment with surgery and chemotherapy is the mainstay of treatment for HB.
Ideal treatment strategy for PRETEXT I tumors is controversial. Some authors have suggested complete resection alone without chemotherapy as an option. This is especially true for the low-risk tumors with fetal histology where complete surgical resection with follow-up has been shown to be effective in providing long-term disease control [13]. Advocates for this approach highlight the fact that these children are spared the adverse effects of chemotherapy such as ototoxicity [14]. The SIOPEL group advocates neoadjuvant chemotherapy for all HB. The purported advantages of this approach are that it shrinks the tumor, and clearly demarcates the tumors making resection more straightforward. Tumors also become more fibrotic and hence intraoperative handling is easier.
The current protocol for PRETEXT II and III is to give up to four cycles of neoadjuvant chemotherapy, followed by assessment for resection (Fig. 69.3).
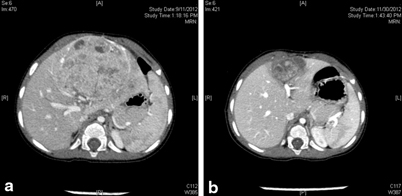

Fig. 69.3
CT images of Hepatoblastoma in a 1-year-child. a CT at presentation showed a PRETEXT 2 disease with tumor involving the two left sectors. b CT following four cycles of chemotherapy prior to resection. Note the significant shrinkage in tumor size. The CT appearance of the tumor has also changed and the demarcation between the tumor and healthy liver tissue is much more clear
If the tumor becomes resectable, then surgery is followed by two more cycles of chemotherapy. If the tumor remains unresectable, two further cycles of chemotherapy may be considered before a decision is made regarding attempted resection or primary transplantation. Monitoring the fall in AFP level after beginning chemotherapy and after surgery is an excellent means of predicting tumor response. The chemotherapy regimen advised by the SIOPEL group varies for the standard risk and high-risk HB (Table 69.7)[11].
Table 69.7
Risk stratification and treatment of hepatoblastoma (SIOPEL guidelines). (Available at www.siopel.org [11])
Risk status | Definition | SIOPEL guideline for treatment |
|---|---|---|
Standard risk | PRETEXT I, II, III without any other risk factors as defined belowa | SIOPEL 3, Cisplatin alone arm |
Cisplatin X 4 cycles | ||
Surgical resection | ||
Cisplatin X 2cycles | ||
High risk | PRETEXT IV or any PRETEXT stage with vascular involvement (P2 or V3), extrahepatic disease (E1, E2), tumor rupture (H1) | SIOPEL 3, SUPERPLADO arm |
Alternating cycles of Cisplatin and carboplatin + doxorubicin X 7 cycles | ||
Resection/Transplantation | ||
Alternating cycles of Cisplatin and carboplatin + doxorubicin X 3 cycles | ||
Very high tisk | Any tumor with metastases or very low AFP (< 100 ng/ml) | SIOPEL 4, dose-dense cisplatin-based chemotherapy or enrolment in clinical trial |
Surgical resection for HB should be carefully planned and is best carried out in units with expertise in pediatric hepatobiliary surgery and liver transplantation (LT). This is particularly true in children with large tumors and borderline resectability. Children can tolerate extensive liver resections better than adults, and up to 85 % of liver can be resected safely. More aggressive liver resection techniques such as total vascular exclusion and caval resection may be required to achieve complete disease clearance.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







