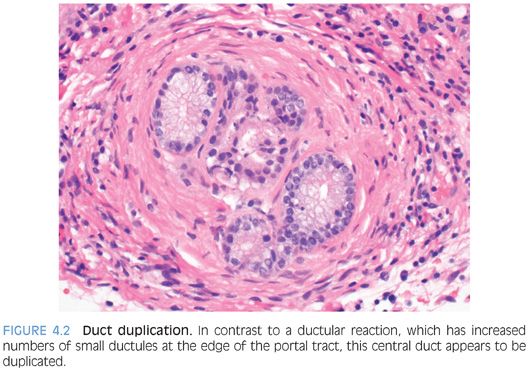
Chronic biliary obstructive disease is most commonly encountered with primary sclerosing cholangitis, chronic pancreatic disease with duct stricturing, or anastomotic strictures after liver transplantation, but other less common causes of strictures or obstruction can also be seen. The biopsies show predominately portal tract changes with bile ductular proliferation and often portal fibrosis. The ductular proliferation is often patchy and can vary in intensity. An accompanying mild chronic portal inflammation is common, composed of lymphocytes with the addition of neutrophils in some cases. Portal tract edema is less common than with acute obstruction. The portal tracts may contain more than one bile duct profile (duct duplication, not to be confused with ductular proliferation) (Fig. 4.2, eFig. 4.1). Other possible changes include loss of bile ducts, cholate stasis, and lobular cholestasis. Fibro-obliterative duct lesions can be seen in cases with long-standing extrahepatic biliary strictures.
Bland Lobular Cholestasis
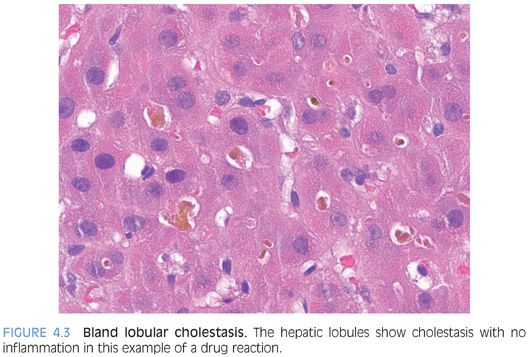
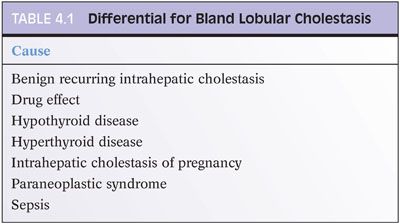
This pattern should have little or no portal tract changes, little or no lobular inflammation, and no significant fatty change. The cholestasis is predominately found in the hepatocytes or bile canaliculi (Fig. 4.3). When presenting as an acute hepatitis, this pattern of injury is most commonly a result of a drug effect. Occasionally, acute viral hepatitis, such as hepatitis E infection, can also show predominately bland cholestasis with relatively little inflammation. Biliary obstruction can also have lobular cholestasis, but the two can be differentiated by the portal tract changes seen in acute biliary obstruction. Likewise, a moderate to severe lobular hepatitis is often associated with lobular cholestasis, but the inflammatory changes provide a clear separation between these entities. Bland lobular cholestasis is also common in patients with sepsis. There are several other important causes of chronic or intermittent liver enzyme elevations with a bland lobular cholestasis pattern (Table 4.1).
Fatty Liver
Fat in the liver can be predominately macrovesicular or microvesicular. The macrovesicular pattern of fat is by far the most common, being a key element in the pathology of both alcohol and nonalcohol fatty liver disease. Steatosis and steatohepatitis are discussed in detail in Chapter 9, but the primary differential is that of alcohol-related liver disease, metabolic syndrome (central obesity, diabetes mellitus, hypertension)–related liver disease, and drug effect. Fatty liver can also be caused by inherited diseases that affect metabolism in infants and children. Malnutrition is another cause but one rarely seen on liver biopsy. Other causes are listed in Table 4.2.
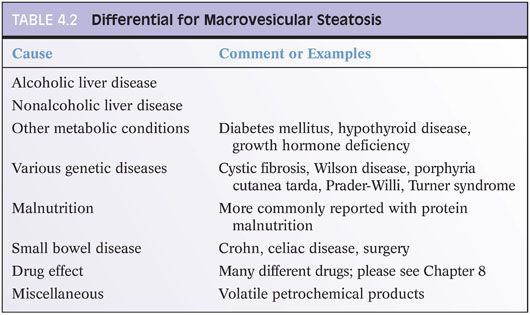
Microvesicular steatosis has a different differential than macrovesicular steatosis. The microvesicular pattern of steatosis shows diffuse liver involvement with hepatocyte cytoplasm filled with numerous small droplets of fat (Fig. 4.4). Small amounts of macrovesicular fat may also be present, but typically, there is relatively little inflammation. This pattern of injury is quite distinctive and results from mitochondrial toxicity. This injury pattern is most commonly seen in the settings of a drug effect, a rare form of alcoholic liver disease called alcoholic foamy liver degeneration, or fatty liver of pregnancy. In infants and children, the differential is focused on mitochondrial defects or other inherited mutations that affect aspects of the urea cycle or fatty acid oxidation. The differential also includes a number of other rare infections or toxin exposures (Table 4.3). The clinical setting in most cases is distinct, and the combination of clinical history and histologic findings leads to a clear diagnosis in most cases. Of note, approximately 10% of typical metabolic syndrome–associated fatty liver disease may have small discrete foci of microvesicular steatosis,8 but this finding should not be interpreted as having the same significance as a diffuse microvesicular pattern of steatosis. Also of note, after a massive liver necrosis from many different causes, ranging from ischemia to acetaminophen injury, the surviving hepatocytes may show microvesicular steatosis. In this setting, the finding of microvesicular steatosis is nonspecific and does not necessarily engender the differential listed in Table 4.3.
Microvesicular steatosis is best diagnosed on H&E stains for clinical purposes, not Oil red O stains. Although older publications have recommended using Oil red O stains on frozen sections to make a diagnosis of microvesicular steatosis,9 this is now realized to be a problematic approach. Extensive experience in evaluating donor liver biopsies at the time of transplantation indicate that diffuse small droplet fat is commonly seen in Oil red O stains performed on healthy livers (eFigs. 4.2 and 4.3). Others have also shown that many different livers affected by various diseases that have nothing to do with microvesicular steatosis will still have extensive small droplet staining with Oil red O staining, having a pattern that is indistinguishable from true microvesicular steatosis.10 Diagnostic misadventures can result from making a diagnosis solely on the Oil red O stain.
Hepatitic Pattern
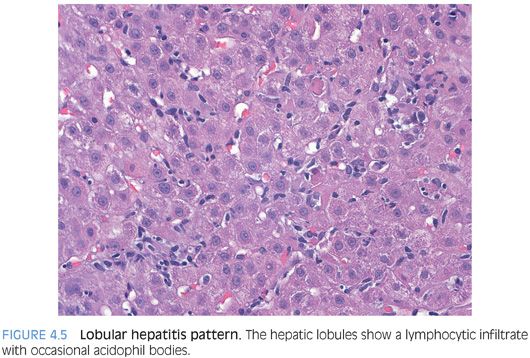
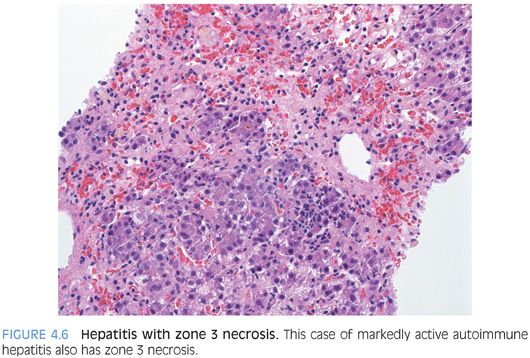
The hepatitic pattern of injury is a very common pattern in biopsy specimens seen in routine practice. The biopsy typically shows lobular inflammation that is composed predominately of T lymphocytes and ranges from mild to marked depending on the severity of the disease (Fig. 4.5). There may be scattered apoptotic hepatocytes, ballooned hepatocytes, and areas of zone 3 confluent necrosis in more severe cases (Fig. 4.6). In particularly severe cases, the necrosis can form bridges that extend from central vein to central vein or central vein to portal tract. The normal lobular organization may be disrupted and the usual neat rows or cords of hepatocytes may be replaced by a more haphazard arrangement of hepatocytes, a find called lobular disarray. Cholestasis may be present, particularly in cases with moderate to severe disease.
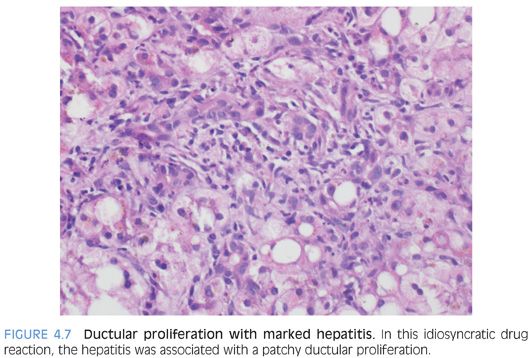
The portal tracts show a predominately lymphocytic infiltrate in most cases, often mixed with smaller numbers of plasma cells, histiocytes, and occasional eosinophils. The lymphocytes will be a mixture of B cells and T cells. In cases of marked acute hepatitis, the portal tracts also may show ductular proliferation (Fig. 4.7). This finding can be an important diagnostic pitfall because the ductular reaction can be sufficiently prominent to suggest downstream biliary tract disease.11 However, in these cases, the moderate to marked lobular hepatitis is usually sufficient to indicate the proper diagnosis.
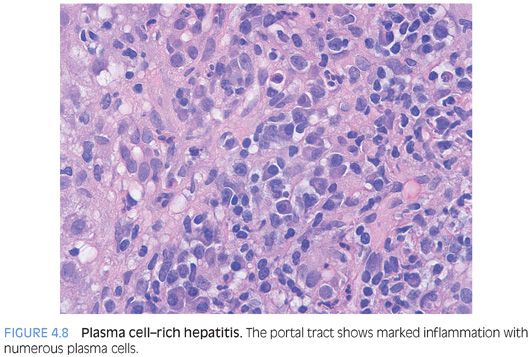
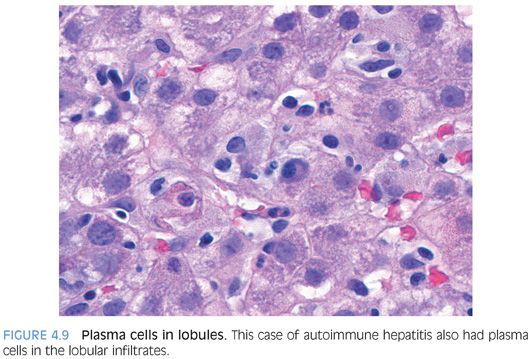
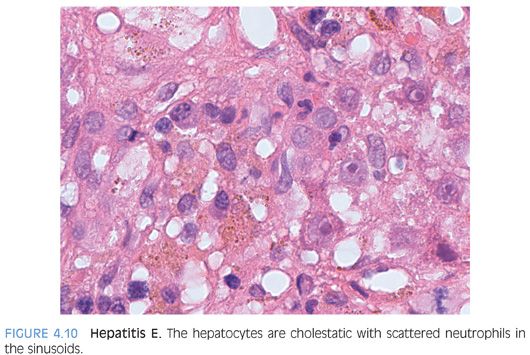
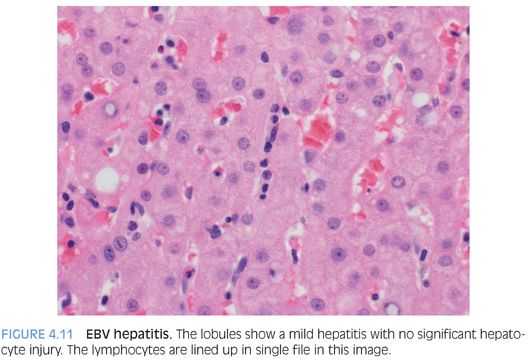
POSSIBLE HISTOLOGIC CLUES TO THE ETIOLOGY. As noted previously, the specific cause of the acute hepatitis will not be apparent in most cases and the general differential is acute viral hepatitis, drug effect, and autoimmune hepatitis. Some findings that will help push the differential in one direction include prominent plasma cells (Fig. 4.8), which are not specific but can suggest autoimmune hepatitis. However, be aware that acute viral hepatitis, particularly hepatitis A or B, can have prominent plasma cells. Plasma cells in the lobules also favor autoimmune hepatitis (Fig. 4.9). Although this finding has not been well validated in formal studies, over the years, the author has personally found it useful. Prominent eosinophils would suggest an allergic type drug reaction, but remember that most drug reactions do not have prominent eosinophils and are in fact predominately lymphocytic in nature. A cholestatic hepatitis with neutrophils in the lobule should suggest the possibility of acute hepatitis E (Fig. 4.10). Sinusoids that are densely packed with lymphocytes, often with relatively mild hepatocyte injury for the amount of lymphocytosis, suggest the possibility of acute Epstein-Barr virus (EBV). The lobular infiltrates in EBV can be patchy and vary in density. Many times, the lymphocytes will be lined up or “beaded” within the sinusoids (Fig. 4.11). Of course, the presence of specific viral inclusions, such as those seen in cytomegalovirus (CMV), is useful in identifying an etiology. There are relatively few other useful findings that will aid in suggesting a specific diagnosis. A zone 3 predominant pattern of hepatitis, often with a mild lymphocytic venulitis, can be seen in autoimmune hepatitis but can also be seen with drug effects and with acute viral hepatitis.
Giant Cell Transformation Pattern
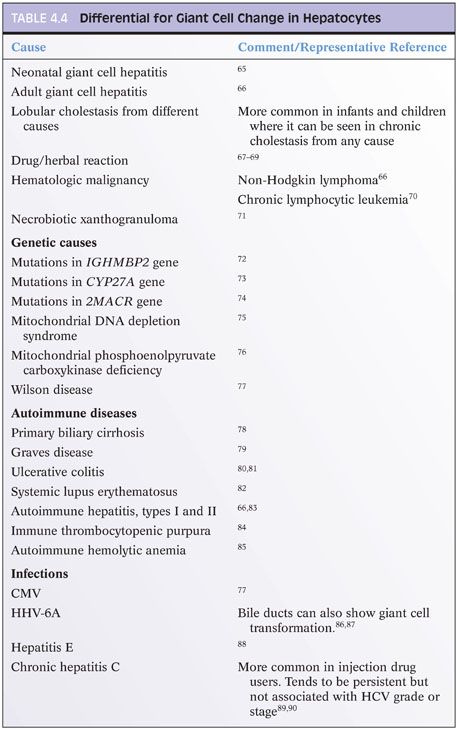
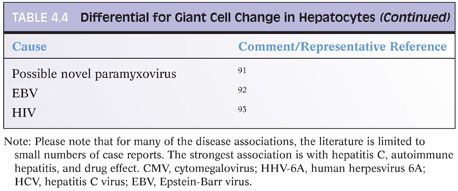
Giant cell transformation is a nonspecific reactive change seen within hepatocytes. When it is the dominant histologic finding, the diagnosis of neonatal giant cell hepatitis or adult giant cell hepatitis is made. However, mild giant cell transformation can be seen in a variety of conditions and does not merit a diagnosis of giant cell hepatitis by its mere presence. The hepatocytes in giant cell transformation are generally nonproliferative and may result from fusion of hepatocytes or from nuclear division without cytoplasmic division. As a focal or patchy mild change, it is most commonly seen in cholestatic conditions. The reason it is seen in some individuals but not others remains obscure. The differential for giant cell change is shown in Table 4.4.
Bland Necrosis
This pattern of injury is associated with toxic injury such as acetaminophen or with ischemia. The hepatocyte necrosis can be limited to zone 3 or can be panacinar with no respect for anatomic land marks. In most cases, there is little or no portal or lobular inflammation (Fig. 4.12). If there has been sufficient time between the injury and the biopsy, the portal tracts may have a significant bile ductular proliferation. The surviving hepatocytes often show small- and intermediate-sized fat droplets. An iron stain may show marked iron accumulation in the Kupffer cells, and if there has been a ductular proliferation, the small ductules may have extensive iron staining. This pattern is most commonly seen with acetaminophen toxicity or other direct toxins.
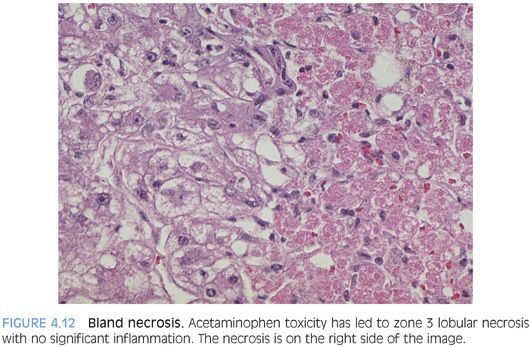
Ischemia can also give the same pattern of injury. Early ischemic injury can be challenging to recognize because the hepatocytes will still be present and many or all may still have their nuclei. However, the cytoplasm of the dead cells is more eosinophilic and distinct color differences will be evident, typically with a zone 3 distribution. This is often more noticeable with the use of a lower power lens such as a 4× or 10× lens. Identifying the acute necrosis pattern of injury on a frozen section can be particularly difficult, but with careful attention, the cytoplasm of the hepatocytes will be noticeably more eosinophilic in the necrotic area and this pattern will guide you to the proper diagnosis. If the liver is cirrhotic, the injury pattern with ischemia will generally not be zonal, but instead, the center of cirrhotic nodules or sometimes scattered entire nodules will be necrotic.
In some cases, the source of injury may be known and the biopsy performed is not for an etiologic diagnosis but to assess the amount of necrosis. In these cases, an estimate of necrosis to the nearest 10% is usually sufficient. If the biopsy is obtained during a laparotomy, remember that the surgeon may have sampled a circumscribed ischemic lesion and thus the biopsy may not be representative of the entire liver. If the biopsied area is a focal lesion, indicate so in the biopsy report.
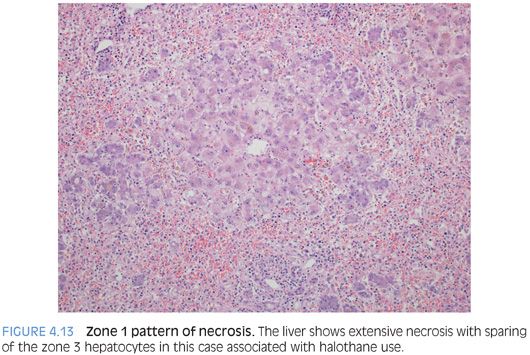
A predominate zone 1 necrosis pattern is a much less common pattern of bland liver necrosis. The necrosis often extends into zone 2 (Fig. 4.13). With more extensive necrosis, the zonal patterns can sometimes be challenging and the overall zonality is often best assessed by looking for where the hepatocytes are still viable. Reported etiologies for zone 1 necrosis include halothane toxicity (more commonly causes zone 3 necrosis), ferrous iron toxicity, white phosphorous toxicity, endotoxin release from proteus vulgaris, and some industrial chemicals such as allyl alcohol. Selective zone 2 necrosis is also very rare. The differential includes rare poisons such as ngaione, heavy metals such as beryllium, and rare viral infections such as yellow fever virus. Of note, both the zone 1 and the zone 2 patterns of liver necrosis are sufficiently rare that many associations are based on data limited to a small set of case reports.
Vascular Injury Pattern
Vascular injury is discussed in detail in Chapter 13, but a useful conceptual framework is to think of vascular injury as having several major patterns. The first is an obstructive pattern, such as with Budd-Chiari syndrome or chronic heart disease, where the main finding is that of congestive hepatopathy and often a zone 3 pattern of fibrosis. A second pattern is that of injury to the endothelium of the central veins, leading to varying degrees of thrombosis and fibrosis. This pattern can be caused by vascular thrombosis due to clotting disorders, chemotherapy-related changes, infection or inflammatory central vein injury, and alcohol-related liver disease. A third pattern is injury to the sinusoidal endothelial cells. This pattern can be seen with viral infections and drug effects, and the main finding is that of sinusoidal dilatation, congestion, and endothelial damage.
Finally, vascular flow–related pathology is also evident with loss of the portal veins. The pathology, in addition to the absence or scarring of the portal veins, may include nodular regenerative hyperplasia or generalized liver atrophy. Liver atrophy can be detected by imaging studies or at the time of surgery, but the histologic correlate is that of portal tracts that are more closely approximated than in a normal liver.
CHRONIC LIVER INJURY PATTERNS
Liver fibrosis usually indicates chronic liver injury. However, there are many cases of chronic hepatitis that may lack fibrosis. These include chronic viral hepatitis, autoimmune hepatitis, drug effects, fatty liver disease, and many others. Thus, the lack of fibrosis should not be interpreted as indicating a lack of chronicity. Specific causes of chronic hepatitis are discussed in their respective chapters, but this section considers findings that may be present in a variety of different types of disease and can be helpful indicators of chronic liver disease. The final section is focused on fibrosis evaluation, including the most important staging pitfalls.
Ductopenia
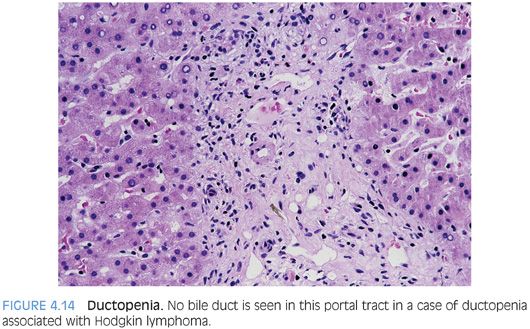
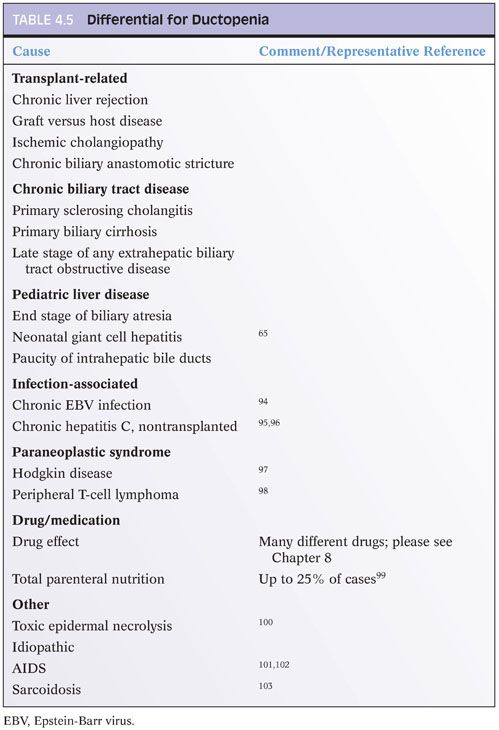
Ductopenia, or loss of intrahepatic bile ducts, is also referred to as the vanishing bile duct syndrome. Ductopenia is most commonly identified in the setting of chronic biliary tract diseases such as primary sclerosing cholangitis or primary biliary cirrhosis. Duct loss is also a defining feature of chronic rejection in the liver allograft. However, ductopenia can also be seen in many other conditions, including paraneoplastic syndromes (Fig. 4.14) and drug affects.12 The full differential is wide and requires a comprehensive review of the biopsy and clinical findings to make the diagnosis (Table 4.5). However, a proportion of cases remain idiopathic despite extensive clinical and pathologic evaluation.13 When present, ductopenia provides strong evidence for chronic liver disease.
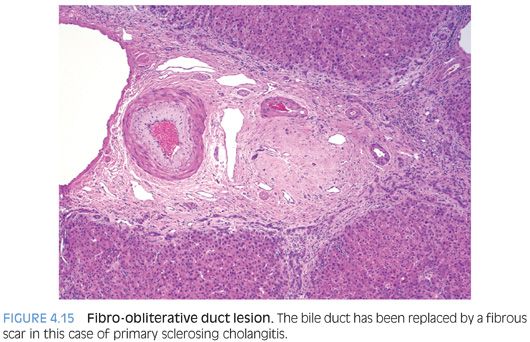
Fibro-obliterative Duct Lesions
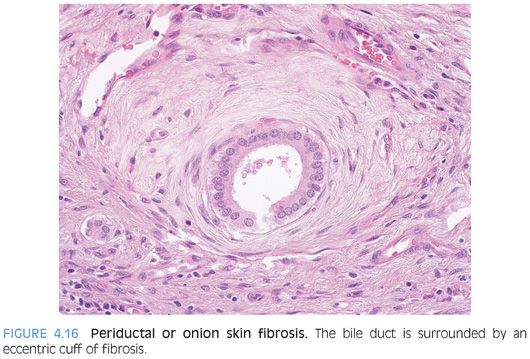
A fibro-obliterative duct lesion is a round or oval fibrous scar that has replaced a bile duct (Fig. 4.15). These lesions can be seen with chronic obstruction of the extrahepatic biliary tree from any cause. They are, however, rather uncommon and will not be seen in most biopsy specimens from cases of chronic obstructive biliary tract disease. In earlier stages of this lesion, the bile duct is surrounded by a dense collar of lamellar fibrosis, a finding termed onion skinning (Fig. 4.16). As a caveat, the normal large-sized bile ducts can have a well-formed ring of fibrosis, and this finding is sometimes mistaken for onion skinning fibrosis. The bile ducts in true onion skinning fibrosis often appear atrophic and this can be a helpful clue.
Hepatocyte Accumulation of Iron or Copper
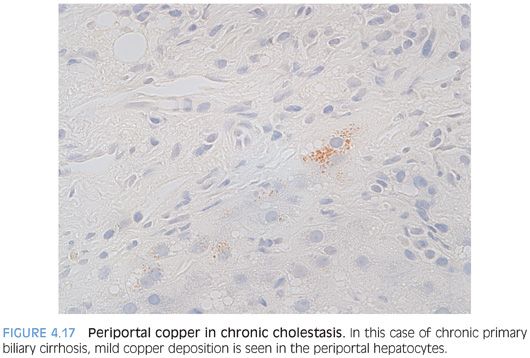
Iron and copper overload are discussed in separate chapters, so they will not be discussed in detail here. Copper accumulation, outside of the setting of Wilson disease, can be a useful indicator of chronic cholestasis in the noncirrhotic liver because copper is normally excreted in the bile. Copper staining in cirrhotic livers is sensitive but less specific for chronic biliary tract disease as the primary disease process and can be seen in a variety of different etiologies.14
Copper accumulation is usually detected with the rhodanine stain. Copper accumulates in primary biliary cirrhosis, primary sclerosing cholangitis, and other chronic cholestatic conditions. The copper is not part of the disease per se but instead is secondary to the chronic cholestasis.15 The copper accumulation is periportal and often focal and mild, so the copper stain has to be examined carefully (Fig. 4.17). Any chronic liver disease that develops chronic cholestasis can show copper accumulation, including drug effects, alcoholic liver disease, and chronic viral hepatitis.16 One study has reported copper accumulation in α1-antitrypsin deficiency, including 50% of noncirrhotic liver biopsies and 100% of cirrhotic biopsies with α1-antitrypsin deficiency.17 Copper accumulation is also seen in most cases of focal nodular hyperplasia due to chronic cholestasis.18 Hepatocellular carcinomas that are cholestatic can also be positive for copper on rhodanine stain.19 Also of note, the normal neonatal liver can be positive on copper stain.20