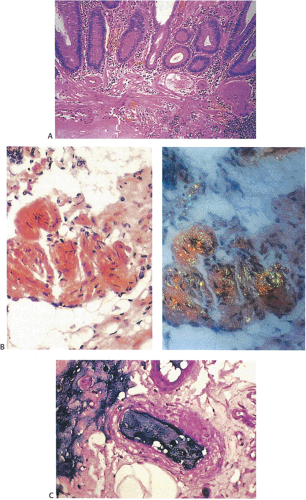
Lesions Presenting as Polyps
Normally, the dynamic process of cell division is balanced by exfoliation from the luminal surface. If an imbalance occurs, either because of increased replication or decreased exfoliation, a polyp results. Broadly speaking, the word polyp simply denotes any mucosal elevation. Polyps may consist of benign or malignant epithelial proliferations, inflammatory infiltrates localized in the lamina propria, or deeper, submucosal or intramural mesenchymal proliferations, malformations, or even metastatic tumors. The three most common colorectal
polyps are inflammatory, hyperplastic, and adenomatous (see Chapter 14).
polyps are inflammatory, hyperplastic, and adenomatous (see Chapter 14).
Hyperplastic Polyps
Hyperplastic polyps are one of the most common polyp types seen in the adult colon. They share risk factors with adenomas and colon cancer including dietary fiber, calcium, and total fat intake; smoking; body mass index; and alcohol consumption. They often arise in a colon harboring adenomatous polyps or carcinomas (576,577). They often cluster in large numbers around colon carcinomas. Hyperplastic polyps increase in frequency with age in some populations, but not all studies confirm this (578,579). The hyperplastic polyp:adenoma ratio is highest in those parts of the world with the highest incidences of colorectal carcinoma and it falls to unity in low-risk regions (580). These lesions remain asymptomatic and are found incidentally at the time of colonoscopy or resection.
The relationship between hyperplastic polyps and the subsequent development of adenomas and carcinomas remains controversial. Hyperplastic polyps have been regarded by most observers as innocuous and nonneoplastic and unrelated to colorectal cancers, whereas others regard them as possible precursors of colorectal neoplasia (581). This issue has been clouded by the recognition of mixed hyperplastic–adenomatous polyps. Mixed polyps combine the features of both hyperplastic and adenomatous polyps without intermediate forms between them. This may be explained by the engulfment of a pre-existing hyperplastic polyp by a spreading adenoma, by stimulation of the mucosa at the advancing edge of an adenoma, or by the development of an adenoma in a hyperplastic polyp. The hyperplastic component alone does not predispose to carcinoma, but the presence of a coexisting adenomatous component does have malignant potential. It is also clouded by the inclusion of the serrated adenomas and the polyps of the hyperplastic polyposis syndrome, which are different entities that somewhat resemble hyperplastic polyps. These lesions are discussed in Chapters 12 and 14. Hyperplastic polyps are generally not regarded as having a direct relationship to colonic cancers (582,583).
The fact that the vast majority of hyperplastic polyps arise in the colons of patients with concomitant adenomas (584) has prompted speculation that hyperplastic polyps are biomarkers of colorectal neoplasia and that the associations are causally related. Some suggest that hyperplastic polyps may be a marker for an environmental factor implicated in the progression of adenomas to carcinomas (585). Alternatively, individuals may be constitutionally prone to the development of both the adenomas and hyperplastic polyps, and they may require similar environmental conditions for their development. The lesions, or at least some of the lesions, may not be as innocuous as once believed since they contain a variety of genetic abnormalities. There is evidence of dysregulated cell proliferation and apoptosis (582,586,587). The presence of clonal genetic alterations, including K-ras (588,589,590), BRAF (590), and TGFβRII mutations; DNA microsatellite instability; and loss of the APC, p53, p16 genes and a tumor suppressor gene on chromosome 1p has led to the suggestion that hyperplastic polyps are “neoplastic” but lack malignant potential (591). There is also methylation of the DNA repair gene O6methylguanine DNA methyltransferase (MGMT) in some hyperplastic polyps (592).
It appears that a subset of “hyperplastic polyps” may be a biomarker of increased risk or even represent a subtype carrying a significant malignant potential (592). These are now commonly referred to as sessile serrated polyps or sessile serrated adenomas as discussed further in Chapter 14. Identical DNA microsatellite alterations can be found in the hyperplastic and adenomatous regions of mixed hyperplastic–adenomatous polyps, suggesting that the two lesions are sequentially related (591). Features that might suggest the presence of a hyperplastic polyp with malignant potential include those listed in Table 13.29 (591).
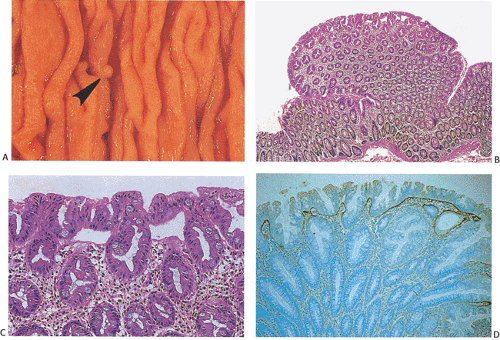
Hyperplastic polyps are pale, sessile nodules usually developing on the crest of mucosal folds. The lesions are often multiple and appear as small mucosal elevations (Fig. 13.164), usually measuring <5 mm in diameter and rarely measuring >1 cm; most arise in the sigmoid and rectum. Their surfaces appear smooth and glistening, and the lesions are usually slightly paler than the surrounding mucosa. Hyperplastic polyps are significantly smaller and lighter in color than adenomas. Endoscopically, it is not possible to distinguish between small adenomas and hyperplastic polyps, so the lesions tend to be biopsied.
Hyperplastic polyps consist of groups of elongated hyperplastic colonic crypts. The upper half of the crypt contains characteristic intraluminal papillary infoldings. These infoldings result from an expanded replication zone and a slower rate of maturation that give the crypt a serrated or saw-toothed appearance (593). While the proliferative zone is lengthened, it is still restricted to the bottom of the crypt. Epithelium lining the crypts contains a mixture of absorptive, goblet, and endocrine cells (594). Absorptive columnar cells predominate over goblet cells. The upper part of the crypts appears crowed by a population of hypermature goblet cells, which are usually larger than normal due to intracellular mucin accumulations. The nuclei remain in a basal
location and are typically enlarged and vesicular with a prominent nucleolus. Atypical mitoses are rare. The bases of the crypts appear hyperchromatic and immature, resembling adenomatous epithelium. However, unlike adenomas, the epithelium matures as one progresses up the crypt toward the lumen. A large proportion of hyperplastic polyps also contain a hybrid epithelium with bidirectional differentiation toward both gastric foveolar and colonic epithelium in the same crypt (595). The replicative zone of the crypt, which contains mitotically active cells, is expanded and may comprise its lower half (Fig. 13.164). A hyperplastic pericryptal myofibroblast sheath associates with the hyperplastic epithelium. It produces an increased amount of collagen, a feature best appreciated under the free surface where there is a thickened collagen table. These lesions may become inflamed and when they do they may appear mucin depleted.
location and are typically enlarged and vesicular with a prominent nucleolus. Atypical mitoses are rare. The bases of the crypts appear hyperchromatic and immature, resembling adenomatous epithelium. However, unlike adenomas, the epithelium matures as one progresses up the crypt toward the lumen. A large proportion of hyperplastic polyps also contain a hybrid epithelium with bidirectional differentiation toward both gastric foveolar and colonic epithelium in the same crypt (595). The replicative zone of the crypt, which contains mitotically active cells, is expanded and may comprise its lower half (Fig. 13.164). A hyperplastic pericryptal myofibroblast sheath associates with the hyperplastic epithelium. It produces an increased amount of collagen, a feature best appreciated under the free surface where there is a thickened collagen table. These lesions may become inflamed and when they do they may appear mucin depleted.
TABLE 13.29 Features that May Indicate “Hyperplastic Polyps” with a Malignant Potential | |
|---|---|
|
Serrated crypts, a hallmark of the hyperplastic polyp, may be seen in the regenerative mucosa in a number of settings including IBD, mucosal prolapse, and juvenile polyps. The major entity in the differential diagnosis of the hyperplastic polyp is the serrated adenoma discussed in Chapter 14. The two lesions are compared in Table 13.30.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree