Fig. 32.1
Robotic technology. (a) The robotic console, (b) proper robotic arm positioning, (c) Robotic instrumentation placed with aid of bedside assistant
Authors of some early robotic colectomy trials have suggested clinical benefits. Robotics may also afford better nerve function after TME. A nonrandomized review of urogenital function after robot-assisted total mesorectal excision for rectal cancer showed faster recovery of normal voiding, erectile function, and sexual desire compared to patients who underwent laparoscopic TME [51]. As laparoscopy did not show improved sexual and urinary dysfunction outcomes over open TME in rectal cancer patients [52], there is hope that robotics might improve these outcomes (Fig. 32.2). A trend toward less postoperative blood loss [49, 53] and early recovery of functional outcomes has been described, although one of these papers was compared to open surgery. Robotic resections have also shown lower conversion rates to open procedures in some series. A recent meta-analysis supported that the conversion to open rate may be reduced with robotics over laparoscopy in both benign and malignant colorectal cases [49, 54–57].
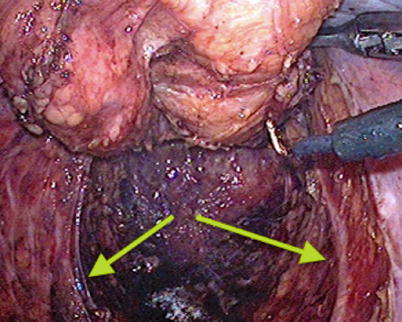

Fig. 32.2
Pelvic hypogastric nerves (arrows) seen on laparoscopy (Courtesy Matthew Mutch, MD)
Robotics may have the most promise in the management of rectal cancer [56, 58]. Results from the MRC-CLASICC trial’s evaluation of laparoscopic versus open surgery for colorectal cancer raised early concerns of adequate TME, risks of higher positive circumferential resection margins, overall male sexual and erectile dysfunction, and worse overall survival in patients converted to open operation [13, 14, 18, 59]. Worse overall survival has not been validated to date. One recent prospective study showed a significantly higher complete mesorectal grade in the robotic versus the laparoscopic group for rectal cancer [57]. At present there are no studies showing a significant benefit in the oncologic outcomes of circumferential resection margin, distal resection margin, or lymph node yield [55, 56, 60], although multiple prospective randomized controlled trials are ongoing to definitively evaluate outcomes for rectal cancer. The ROLARR trial [61], a worldwide superiority trial of robot-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer, is currently underway. The ROLARR trial is investigating differences in rate of conversion to open operation, rate of pathological involvement of circumferential resection margin, 3-year local recurrence, disease-free and overall survival rates, and also operative morbidity and mortality, quality of life, and cost-effectiveness. The ACOSOG Z6051 trial is also underway, comparing outcomes between minimally invasive and open rectal resection, including pure laparoscopic, laparoscopy-assisted, robot-assisted, or hand-assisted methods in the minimally invasive group. Results of these trials will help guide the future role of robotics in rectal cancer (Table 32.1).
Table 32.1
Early outcomes from robot-assisted colorectal surgery
Author | N | Conclusion |
|---|---|---|
Diverticulitis | ||
Zimmern [62] | 16 | Safe |
Low conversion rate | ||
Abodeely [63] | 22 | Safe |
No conversion, no leaks | ||
Ragupathi [64] | 24 | Safe (complicated diverticulitis) |
No conversions | ||
Low complication rate | ||
Rectal prolapse | ||
de Hoog [65] | 20 | Safe |
High recurrence rate | ||
Zimmern [62] | 8 | Safe |
Abodeely [63] | 10 | Safe |
Bokhari [66] | 5 | Safe |
Right hemicolectomy | ||
de Souza [67] | 40 (vs. lap) | Safe |
Outcomes comparable to lap | ||
Higher cost with robotics | ||
Longer procedure time with robotics | ||
Luca [68] | 33 (vs. open) | Oncologic outcomes similar |
Increased EBL with open | ||
Reduced LOS with robotics | ||
Higher cost with robotics | ||
Longer op time with robotics | ||
The future use of robotic technology in non-prostatic surgery will be determined as time goes on. For colorectal surgery, most studies show similar outcomes to straight laparoscopic colectomy [69–71]; however, long-term outcome data is needed. In several meta-analyses, no advantage was reported in days to passing flatus, LOS, complications, oncological outcomes, anastomotic leakage, or postoperative morbidity and mortality, suggesting equivalent safety [49, 54–56, 60]. Operative times and costs are routinely increased by robotics. Although robotic colorectal surgery may facilitate a reduction in conversion to open surgery, the trials currently in process will help elucidate this finding. Similarly, prospective data are required to support the ability of the robot to improve nerve function and mesorectal grade after TME. The cost implications of any improvements will require evaluation. Overall, robotic surgery for colon and rectal cancer appears feasible and safe; however, the current literature only evaluates short-term outcomes, and data on local recurrence and survival is awaited.
Other issues related to the immature technology deserve attention. The costs are immense with no proven benefit to justify the additional expenditure at present. At a price of more than $1.7 million per robot, $125,000 in annual maintenance costs, and up to $2,000 per case for the cost of single-use instruments, robotic surgery is the most expensive approach. Barbash and colleagues reported if robot-assisted surgeries completely replace conventional surgeries, as is the trend in prostatectomy, an additional $1.5 billion in additional health-care costs would be generated annually – more than $2.5 billion when including the amortized costs of the robots [72]. A recent Journal of the American Medical Association study evaluated the uptake of robotically assisted hysterectomy, costs, and complications compared to the laparoscopic approach [73]. In reviewing nearly 265,000 women who underwent hysterectomy between 2007 and 2010 for benign gynecologic disorders, the authors found robotically assisted hysterectomy dramatically increased from 0.5 to 9.5 %. The robotic cases added an average of $2,189 per procedure, compared to traditional laparoscopic surgery, without any significant benefit in outcomes or complications. Looking at the growth trend, the authors found using robotics for all routine hysterectomies would add an unnecessary $1 to $1.9 billion in unnecessary health-care costs each year [73].
Aggressive marketing may be a factor for the continued growth. Both industry reports and the American College of Gynecologists president noted many patients are learning about the claimed advantages of robotic surgery from widespread marketing hype and an aggressive salesforce [74, 75]. To examine if hospitals are misleading patients about the benefits of robotic surgery to increase patient volume, Jin et al. performed a systematic analysis of 400 US hospital websites. The authors found 41 % described robotic surgery; of those, 78 % used manufacturer-provided stock images, and 33 % linked directly to the manufacturer’s website. Unsupported claims of clinical advantages (86 %) and improved cancer control (32 %) were also found, while no sites mentioned risks of robotic surgery. The authors concluded hospitals overestimate benefits, underestimate risks, and are strongly influenced by the robotic system manufacturer [76].
A learning curve is always present when any new technology is introduced, during which an increase in complication rates can be expected. With robotics, there is no expert consensus on how much training is needed despite rapidly expanding use [72]. Expectantly, major complication data and legal issues are mounting. A series of liability cases against Intuitive Surgical have begun litigation, exposing the company’s failure in its commitments to properly train surgeons to use the da Vinci robotic surgery suite safely [74]. With these issues, investor anxiety is growing. An industrial research report on Intuitive Surgical questioned the company’s stock price and market position given the lack of clinical evidence of superior surgical outcomes and gathering storm of legal liability from failure to adequately disclose risks leading to surgical complications [77].
These factors have culminated in the American College of Gynecologists President James T. Breeden’s statement against the routine use of robotics. Dr. Breeden highlighted an absence of strong evidence that robotic hysterectomy is even as good as, and far more costly than, minimally invasive surgical techniques for routine surgical care. Aggressive direct-to-consumer marketing may mislead the public into believing that they are the best choice. Patients should be advised that robotic surgery should be reserved for complex, specific conditions [75].
Single-Incision Laparoscopy Surgery
Key Concept: Single–incision laparoscopic colectomy provides the potential for improved cosmesis, postoperative pain, and recovery time at the drawback of higher costs, operating time, and technical skill required.
Single–incision laparoscopic surgery (SILS) was introduced to further the enhanced outcomes of traditional laparoscopy. SILS was first reported in 1999 for cholecystectomy [78] then extended to laparoscopic colectomy in 2008 by Remzi et al. [79] and Bucher et al. [80]. SILS uses a single port within the umbilicus with three or more working channels incorporated in the single port. Straight or articulating instruments are used via a fixed platform or small low-profile adjacently placed trans-fascial trocars, theoretically allowing intracorporeal triangulation of parallel instruments (Video 32.2, video by Virgilio George, MD). Studies have proven SILS is feasible and safe [81–86]. From early reports, SILS has similar postoperative outcomes and complications to traditional laparoscopic surgery. Operative time, conversions, estimated blood loss, surgical site infection, and hospital readmissions were all similar [87]. Although some reports noted longer operative times, the results are generally comparable with conventional LC. SILS even has demonstrated benefits over traditional laparoscopic surgery, including better cosmesis, reduced pain, and faster recovery [88]. The cosmetic benefit of a single incision is a major draw. The potential advantages of a small skin incision include not only better cosmetic result but also a lower rate of port-site-related complications (Fig. 32.3) [89]. Another reported advantage of a single incision is less postoperative pain than conventional LC [87, 90]. The reduction in pain translated to lower total narcotic use was in the immediate postoperative period, with lower pain scores reported up to postoperative day 2. SILS has also shown a significantly shorter length of stay (LOS); studies have demonstrated LOS more than 1 day shorter for SILS compared to multi-post-laparoscopy (Table 32.2) [81, 87].
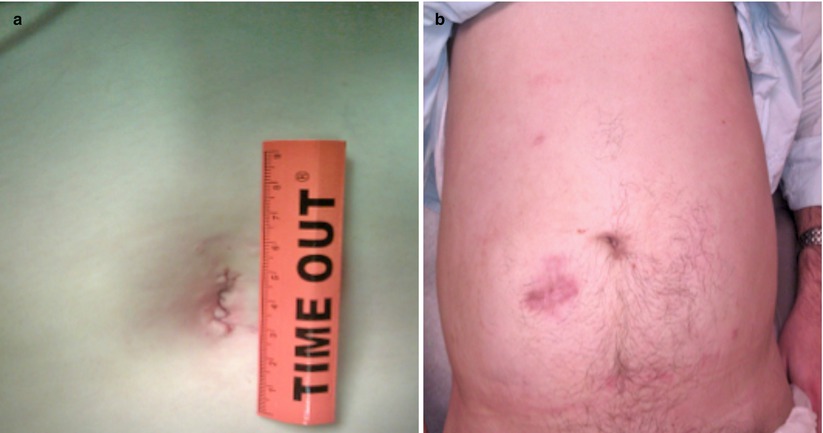

Fig. 32.3
Cosmetic comparison for total colectomy through (a) SILS and (b) traditional laparoscopic approach
Table 32.2
Published reports of single-incision colectomy
Author | Year | Patients | BMI | Mean OR time (min) | LOS (d) | Incision length (cm) | R/L |
|---|---|---|---|---|---|---|---|
Bucher [80] | 2008 | 1 | N/A | 158 | N/A | 3 | 1/0 |
Remzi [79] | 2008 | 1 | 35 | 115 | 4 | 3.5 | 1/0 |
Rieger | 2009 | 7 | 24.3 | 89 | 5.4 | 3.1 | 6/1 |
Geisler | 2009 | 1 | 24 | 172 | 4 | 2 | TPC |
Merchant [91] | 2009 | 1 | N/A | N/A | 3 | 2.5 | 1/0 |
Remzi | 2009 | 1 | 25.8 | 198 | 3 | 3 | 0/1 |
Bucher | 2009 | 1 | 26 | 213a | N/A | 2 | 0/1 |
Law | 2009 | 1 | N/A | 180 | 3 | 3 | 0/1 |
Chambers [88] | 2009 | 6 | N/A | 82 | 1.9 | 2.5 | b2/1 |
Leroy | 2009 | 1 | 21 | 90 | 4 | 2 | 0/1 |
Bucher | 2010 | 1 | 22 | 125 | 2 | 2 | 0/1 |
Adair [83] | 2010 | 17 | 26.2 | 139 | 5 | 3 | 17/0 |
Gandhi | 2010 | 24 | 28.5 | 143 | 2.7 | 3.8 | 19/5 |
Papaconstantinou [81] | 2011 | 29 | 30 | 128.8 | 3.4 | 4.9 | 29/0 |
Chen [85] | 2011 | 18 | 23.3 | 175 | 5 | 4 | 18/0 |
Fichera | 2011 | 10 | 21.9 | 139 | 5.1 | – | TPC |
McNally | 2011 | 27 | 27 | 114 | 3 | – | 14/8c |
Wu | 2011 | 27 | – | 180 | 7 | 4.1 | 8/18d |
Ross | 2011 | 39 | 25.6 | 120 | 4.4 | 4.2 | 30/9 |
Ramos-Valadez | 2012 | 20 | 27.7 | 159.2 | 3.2 | 3.3 | 0/20 |
Walters | 2012 | 100 | 26 | 105 | 4 | 100/0 |
Despite the benefits, some issues exist with SILS. The proximity of the trocars at a fixed position, restricted freedom of the hands, and clashing of the instruments is somewhat contradictory to the traditional teaching of instrument triangulation in laparoscopy [91]. The problems in exposure and the risk of “crowding” while maneuvering laparoscopic instruments add to the difficulty in the SILS technique [92] (Video 32.3, video by Virgilio George, MD). An additional learning curve is involved for the technique, extra incisions are sometimes required [82, 93], and there is a minor increase in cost over laparoscopic surgery [84, 94]. SILS may also make teaching more difficult. Previous studies have demonstrated unique requirements of SILS, with skill sets and ergonomic demands which cannot be directly adapted from existing LAP experience [95]. Thus, the implementation of an evidence- and competency-based SILS curriculum is necessary to ensure appropriate training of future SILS surgeons. Currently, resident training modes are in development for SILS, and the attending may be performing more of the case, at the expense of resident education. Many of these issues can be improved with operator ascension up the learning curve and refinement of the SILS technology. Robotic-utilizing surgeons feel that robotics may also reduce the negativities of SILS such as loss of triangulation and poor visualization and further advance the technology.
Evolving Endoscopic Techniques
Endoscopic Mucosal Resection (EMR)
Key Concept: EMR provides en bloc or piecemeal removal of premalignant and early colorectal lesions typically <20 mm that may have otherwise required resection.
Advanced endoscopic technology has been introduced to allow for treatment of colorectal tumors without the morbidity of a surgical resection. These endoscopic techniques have permitted more aggressive and successful polypectomy, including en bloc removal of otherwise unresectable lesions [96]. Endoscopic mucosal resection (EMR) is an option for endoscopic polypectomy of colorectal polyps without stalks. EMR differs from standard snare polypectomy by the use of submucosal solution injection, which allows for the complete resection of the mucosa through the mid to deep submucosa [97]. EMR is useful for the removal of adenomas that are too large for standard snare polypectomy and essentially allows removal of colonic lesions in a minimally invasive way that would otherwise require surgical colectomy (see Video 25.1) [98]. Although not an absolute contraindication, it is typically more difficult to remove tumors >20 mm by en bloc resection using EMR, with reported success rates of ~30 %; thus, decisions should be made on an individual basis [99–101]. Piecemeal excision (while limiting the full extent of final pathological analysis) can also be used to facilitate removal of larger lesions to a large extent in experienced hands [102].
To perform EMR, the lesion is oriented to maximize the influence of gravity, then a submucosal injection creates a fluid “cushion” between the mucosa and muscularis propria to elevate the lesion into the lumen. Following the injection lift, a snare is deployed to fully remove the lesion with a 2–3 mm margin of normal mucosa [102]. Because the plane of resection during EMR is typically the middle to deep submucosal layer, compared with standard polypectomy, which normally provides resection at a mucosal level, EMR offers the advantage of providing en bloc resection specimens.
Outcomes for EMR are very good for experienced providers. A meta-analysis and systematic review of successful en bloc resections of large colorectal polyps by EMR found complete cure rates improved from 44.19 to 69.17 %, concluding EMR is an effective technique and offers an alternative to surgery [103]. An Australian study of EMR in 174 patients with difficult polyps reported a 95 % procedural success, 90 % avoided the need for surgery, no perforations, and significant cost savings compared to surgical resection [104]. The most frequently reported major complications – perforation (0–5 %) and bleeding (0.5–6 %) – may require surgical management, and removal of large sessile lesions is technically demanding, often requiring a lengthy procedure time to retrieve fragments of lesions and may require multiple endoscopic sessions for complete ablation of a large adenoma [98].
Endoscopic Submucosal Dissection (ESD)
Key Concept: ESD provides an improved ability for en bloc resection over EMR and is a better option for larger superficial colorectal tumors; however, it is technically demanding and has a higher rate of complications.
Endoscopic submucosal dissection (ESD) was developed to overcome the limitations of conventional EMR. ESD is primarily used in Japan and in select centers in Europe and the USA to resect larger polyps and selected invasive tumors and aid in achieving higher rates of en bloc resection of superficial tumors than EMR. ESD is a complicated technique for treating large superficial colorectal tumors because it provides a higher en bloc resection rate and is less invasive than surgical resection. Others have proposed that this technique is suitable for all large polyps, early colorectal cancer, and those lesions that cannot be accessed by transanal or TEMS routes and wish to avoid major resection. ESD can be considered in lesions that have a higher rate of submucosal infiltration and require detailed histopathologic diagnosis by en bloc resection or when fibrosis has developed on the submucosal layer from biopsy and EMR is difficult because of non-lifting signs [105].
The technique of ESD involves an endoscope with a single channel, along with a high-frequency generator (Video 32.4, video by Peter Marcello, MD). After identification of a lesion, a mixture of 1 % hyaluronic acid solution and 10 % glycerin solution is injected around the lesions to elevate the submucosa [106]. The border of the tumor is initially marked by indigo carmine dye with 1 cm margins. Following a mucosal incision, a partial or circumferential incision is made with injection of hyaluronic acid solution into the submucosa, and the dissection is carried down to the deep submucosa. This process is continued around the tumor until the entire lesion is resected en bloc [107]. The en bloc excision with ESD has a number of theoretical advantages, including more accurate histologic assessment, reduced recurrence, decreased endoscopic surveillance requirements, and potential surveillance cost savings [102]. For laterally spreading rectal tumors, ESD is becoming more prevalent, although transanal endoscopic microsurgery is still frequently used [108]. ESD also has the additional advantages of minimal invasiveness and avoidance of anesthesia [109]. Successful en bloc resection has been reported in up to 85–89 % of cases, with piecemeal resection in the remaining 10–15 % [100, 105, 110–112]. However, there are risks with this new technology. ESD is still associated with higher perforation rate, longer procedure times, and increased technical difficulty [113]. The thinner colorectal wall and winding nature of the colon make colorectal ESD an especially difficult operative technique [114]. Further, residual disease has been reported in 2–3 % with ESD [115]. The application of colorectal ESD needs to be further evaluated, with improvements in technology in the technical skill, and surgical devices are required before widespread use.
Endoscopic techniques have evolved to the point where they can be applied to full-thickness resection of polyps, reducing risk compared to surgical resection and accelerating patient recovery. The Tissue Apposition System (TAS) was developed to facilitate this approach (Video 32.5). TAS is a novel endoscopic suturing system that enables endoluminal full-thickness closure [96]. The polypectomy site is closed under laparoscopic observation to avoid injury to surrounding structures. In a feasibility study, TAS was demonstrated to be safe under laparoscopic guidance [96]. Initial studies have shown no long-term complications and normal healed mucosa with the sutures and anchoring devices in place at follow-up colonoscopy [116]. TAS may increase the number of patients whose difficult polyps can be removed endoscopically, avoiding the need for a surgical resection in select patients. Based on early results, TAS sets the future direction in minimizing surgery for endoscopically unresectable colonic polyps. These endoscopic technological advances are improving lesion assessment and standardization, and new methods and techniques are being developed to enhance procedural safety and efficacy.
Combining Laparoscopy and Endoscopy
Key Concept: Combining colonoscopy with laparoscopy allows removal of select previously inaccessible polyps without the morbidity of a surgical resection. Additionally, a standard resection can be performed at that time given advanced pathology, technical problems, or an inability to perform endoscopic removal.
Adding the laparoscopic approach to endoscopically unresectable polyps enriches the therapeutic spectrum. Due to location or size, some polyps are deemed unsafe or technically impossible to treat endoscopically and require colectomy. The perceived risk of iatrogenic injury including hemorrhage and colonic perforation may prevent an attempt at polypectomy [117]. In such cases, where standard polypectomy via the colonoscope is considered not technically possible, patients may be referred for colonic resection. However, there is significant morbidity associated with a surgical resection, including wound infection, anastomotic leak, ileus, and death [3, 5, 10, 18, 118]. By combining laparoscopic mobilization of the bowel with colonoscopic polypectomy – combined laparoscopic and endoscopic resection (CLER) – previously inaccessible polyps could be snared, and laparotomy with enterotomy or bowel resection can be avoided (Fig. 32.4; Video 32.6). Franklin et al. reported on a series of 110 patients undergoing colonoscopic polypectomy following laparoscopic mobilization of the colon [119]. Smaller studies have also demonstrated the feasibility of CLER technique for small series of unresectable polyps [120–124]. A 10-year review of CLER for noninvasive or benign colorectal polyps found low rates of conversion (5 %), major postoperative complications, and intraoperative complications (1 %). However, follow-up colonoscopy revealed metachronous adenomas in more than one-third of patients [125]. The authors concluded that CLER is an efficient, safe, and minimally invasive alternative to open resection for selected patients with difficult polyps. Further experience and results of large-scale trials are needed before applying CLER more broadly. Above all, it is imperative to have the polyp assessed by an experienced endoscopist before embarking on a CLER as this may save the patient from undergoing general anesthesia. The majority of polyps are still possible to remove by standard endoscopic techniques.