Fig. 24.1
Hepatic carcinoma in Couinaud II and III, with cirrhosis
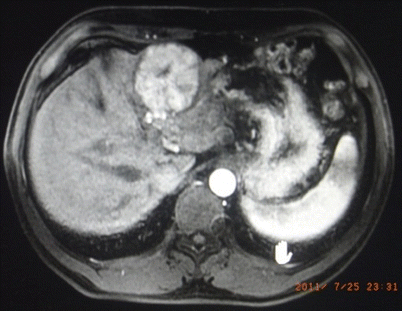
Fig. 24.2
Hepatic carcinoma in Couinaud III
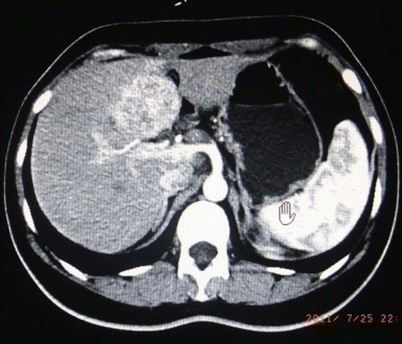
Fig. 24.3
Hepatic carcinoma in Couinaud IVb
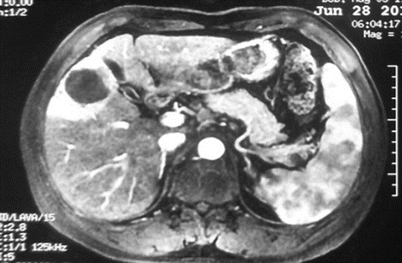
Fig. 24.4
Hepatic carcinoma in Couinaud V, with cirrhosis
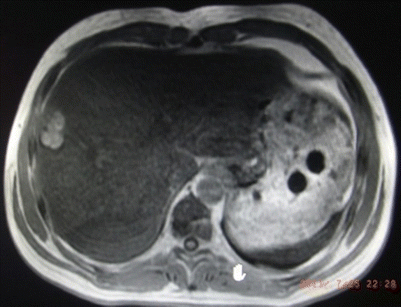
Fig. 24.5
Inflammatory pseudotumor in Couinaud V
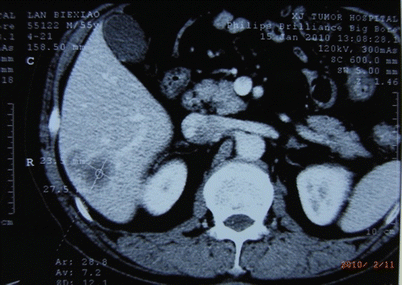
Fig. 24.6
Hepatic carcinoma in Couinaud VI
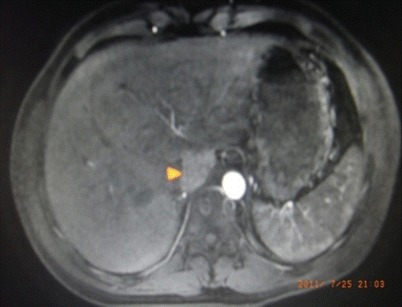
Fig. 24.7
Hepatic carcinoma in Couinaud I
24.3 Contraindications
The contraindications of laparoscopic liver resection include (I) contraindications of open hepatectomy; (II) contraindication of pneumoperitoneum; (III) patients with tumor extension to the hilum, central hepatic veins, or inferior vena cava; (IV) extensive intra-abdominal adhesions; and (V) the need for complex vascular or hepatobiliary reconstruction or extensive lymphadenectomy which should generally be approached as a hybrid or an open procedure (Table 24.1).
Table 24.1
Indication and contraindication to laparoscopic liver resection
Lesions | Indication | Contraindication | |
|---|---|---|---|
Relative | Absolute | ||
Size | <5 cm | >5 cm | |
Location | Segments II, III, IVb, V, VI | Segments I, IVa, VII, VIII | Invading vasculature |
Number | Solitary or multiple in same lobe | More than would be necessary to maintain adequate residual liver function | |
Vascular concerns | Distant from any vasculature | Near inferior vena cava, hepatic veins, hilum | Insufficient oncologic margin |
Pathology | Benign or malignant hepatic neoplasms parasitic lesions | ||
Child class | A | B | C |
24.4 Signs for Conversion
Extensive intra-abdominal adhesions causing difficult dissection and severe bleeding or rupture of digestive tract.
Tumor with excessive size or inappropriate location and difficult exposure of primary and secondary porta hepatis.
Massive hemorrhage, especially for cirrhotic patients. For patients with benign tumors such as hemangioma, most of whom have good liver function, the operation can be continued with blood transplant; however, an amount of 800 ml should be considered as the warning value, and conversion should be forced when blood loss extends to 1500 ml [48].
Injury of extrahepatic veins, failed in fast management, to prevent gas embolism [49].
Sudden hemorrhage and rupture of large vessels or tumor.
Combined with intrahepatic metastasis, cancer embolus in portal vein, hepatic lymph node metastasis, or unclear margin.
24.5 Preoperative Preparation
24.5.1 Patient Preparation
A thorough medical history should be asked and physical examination be performed to evaluate the disease or previous abdominal incisions that might complicate the laparoscopic approach. Severe cardiac, pulmonary, or renal disease should be further evaluated. High-quality magnetic resonance or computed tomography imaging with vascular reconstruction should be reviewed to evaluate intrahepatic arterial and portal anomalies and to determine if the lesion is suitable for a laparoscopic resection. Magnetic resonance cholangiopancreatography is suggested before laparoscopic hemihepatectomy to learn enough information about the biliary system, especially congenital variation.
Informed consent should include a thorough discussion of the risks and benefits of laparoscopic surgery relative to those of open surgery, as well as the possibility of conversion to an open resection.
Patients are recommended to be admitted in no more than 7 days before the operation to conduct routine lab examinations including liver function tests, basic metabolic profile, complete blood count, coagulation series, and tumor markers.
24.5.2 Equipments and Instruments
Improved instruments have greatly improved the safety of LLR. Thus, it is critically important to be familiar with the relevant laparoscopic instruments and equipment. State-of-the-art equipment is required for LLR, and operating room nurses should be familiar with the proper setup of the equipment. The equipment required for LLR includes high-resolution electrical or optical laparoscopic system, 30° laparoscopes, video and picture collection/storage equipment, automatic high-flow insufflation unit, irrigation and suction devices, and instruments for conducting the operation.
A 10 mm tangential clamp should be prepared in laparoscopic regular liver resection, for the dissection of hepatic artery and portal vein in the porta hepatis. The video should be stored routinely.
Generally, the use of two monitors and 10 mm 30° laparoscopes is recommended, which provides better visualization. High-definition monitors are positioned laterally to each shoulder and above the patient’s head. To date, CO2 pneumoperitoneum is considered safe. In many high-volume centers, LLR was performed at a pneumoperitoneal pressure less than 12 mmHg, and reports indicated that the rate of clinically severe gas embolism was low [49].
For the dissection of liver parenchyma, we suggest the combination use of three instruments: ultrasonic dissectors, BiClamp, and argon beam coagulator. Endoscopic linear cutter stapler should also be prepared.
The ultrasonic dissectors are necessary, which work through a vibrating blade or scissor and can be used for tissue dissection and coagulation and mostly were used for the dissection of liver parenchyma and detailed dissection in porta hepatis. It can effectively seal small vessels and bile ducts with minimal fogging of the camera lens and seldom adhere to the liver parenchyma as conventional electrocautery does. Compared to the crushing instruments for parenchymal dissection, the use of the ultrasonic dissector is beneficial because of less hemorrhage during dissection of liver parenchyma. Ultrasonic dissectors allow complete clearance of the liver parenchyma several millimeters around the pedicles, which ensures safe ligature. For the approach to the hepatic veins, ultrasonic dissection allows precise dissection without traction, minimizing the risk of tearing the fragile wall of the hepatic veins.
BiClamp is generally used in the coagulation of active bleeding in the liver incisional surface [50], and argon beam coagulator is generally used in the coagulation of capillary hemorrhage. The argon beam coagulator (ABC) is also useful for hepatic resections, primarily for superficial hemostasis. However, the appropriate use of the ABC system is very important in order to avoid the life-threatening complication of argon gas embolism.
LigaSure of 5 mm could also have a good performance in dissecting liver parenchyma.
Surgical clamps or adsorbable clamps can be used in the ligation of large vessels.
Endoscopic disposable clip appliers and vascular staplers can contribute to the reduction of major intraoperative bleeding during laparoscopic hepatectomy. Because of their safety, rapidity, and ease of application, these stapling devices are efficient in controlling and dividing the major hepatic veins. The size of nail box should be selected according to the thickness of tissue; generally, a nail box with a height of 3.5–3.8 mm and a width of 60 mm is used, and the brand name should be in coincidence with that of trocars. Titanium endoclips can be used to close the main vascular branches and bile ducts. Small vascular or biliary ducts are closed by bipolar coagulation or endoclips. Medium-sized hepatic veins and bile ducts in Glisson’s sheath should be clamped with a clip.
Laparoscopic flexible ultrasonography is not only useful but also indispensable for precisely locating the boundaries of the tumor and the exact anatomy of the vessels, mainly the hepatic veins. Its guidance ensures the safety of both regular and irregular resections. The precise localization of hepatic vessels with color Doppler expands the indications of laparoscopic surgical resection [51, 52].
Gasless laparoscopy is an alternative to the use of CO2 pneumoperitoneum and the abdominal wall lift device. It provides a tent-shaped operative field rather than the more spacious dome-shaped field provided by a pneumoperitoneum [53]. Intra-abdominal organs are closer to the laterally situated port sites, which increases the risk of injury and limits work area. However, gasless laparoscopy avoids the rapid changes in intra-abdominal pressure that are associated with a greater risk of gas embolism. Maintaining intra-abdominal pressure equal to that of the ambient environment may minimize this risk, especially when the hepatic vein is lacerated intraoperatively. Unfortunately, the exposure with the gasless approach is somewhat unsatisfactory. This laparoscopic procedure is recommended for liver cirrhosis patients with small HCC who are not candidates for major hepatectomy [54].
The bag is suggested, especially for malignant cases, to prevent port implant metastasis.
24.6 General Principles of Surgical Technique
24.6.1 Patient Position and Trocar Placement
After patient and procedure confirmation, general anesthesia is induced and a central venous catheter, an arterial line, and a large bore peripheral intravenous catheter, nasogastric tube, and Foley catheter are placed. Routine anesthesia monitoring includes heart rate, arterial blood pressure, oxyhemoglobin saturation, and blood gas analysis.
The patient’s position and trocar placement are decided on the basis of the location of the tumor. Laparoscopic hepatectomy is generally performed with a four- or five-trocar technique.
Generally, the patient is positioned on the operating table in a supine position with the head higher than the feet, and the surgeon stands on the right side of the patient. A 10 mm trocar and 30° laparoscope, which provides a wide-angle view of the operative field, are placed below the umbilicus. After pneumoperitoneum is created by infusion of carbon dioxide, more trocars are inserted at the epigastrium and the bilateral subcostal lines for dissection.
When hepatic resection is performed on the anterior-inferior segment (segment V) or the posterior-inferior segment (segment VI), the patient is placed on a left-sided semireclining position. After pneumoperitoneum is created, three more trocars are inserted at the right subcostal line, the anterior clavicular line, and the epigastrium.
The placement of trocar in laparoscopic surgery is extremely important because it is directly related to the difficulty of the surgery. Four schematic diagrams of routinely used protocols of trocar placement are followed.
Schematic diagram of routinely used protocols of trocar placement
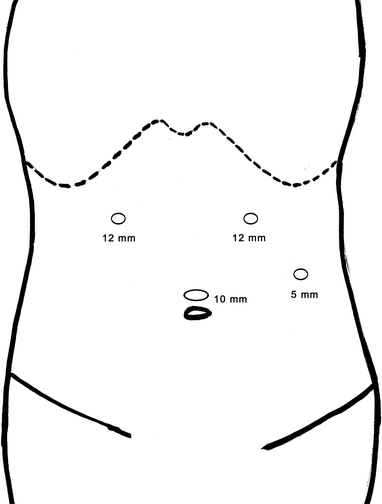
Fig. 24.8
Laparoscopic left lateral sectionectomy
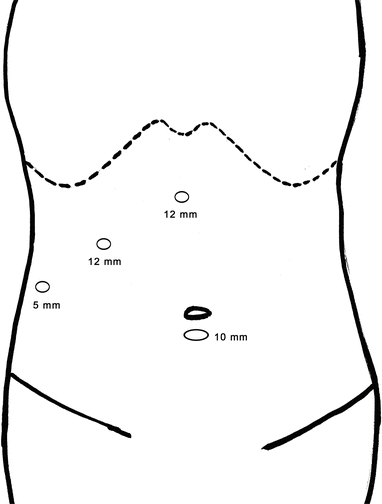
Fig. 24.9
Laparoscopic right posterior partial liver resection
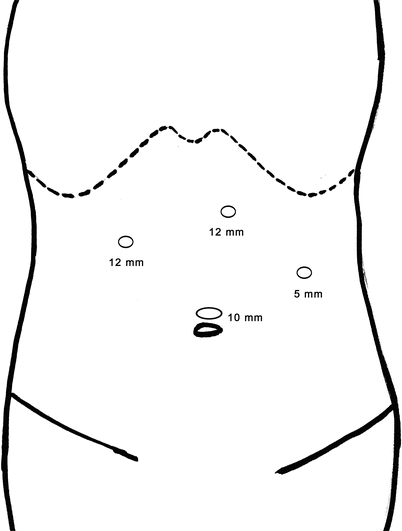
Fig. 24.10
Laparoscopic left hemihepatectomy
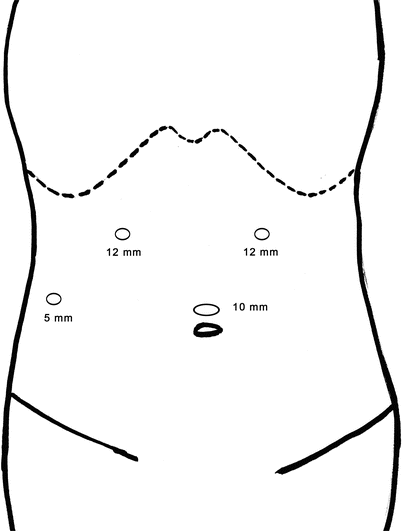
Fig. 24.11
Laparoscopic right hemihepatectomy
24.6.2 Mobilization of Liver and Control of Bleeding
Liver mobilization requires freeing the liver’s ligamentous attachments from the diaphragm. The ligamentum teres hepatis and the falciform ligaments are taken down from the abdominal wall longitudinally to the confluence of the hepatic veins and vena cava. It is important that these ligaments should be transected close to the abdominal wall as to prevent dangling remnants from obstructing the view or soiling the scope.
The left triangular and coronary ligaments are divided close to the liver laterally to medially, similar with the method of dividing the attachment of the lesser omentum. If a replaced or accessory left hepatic artery is present, it should be transected between clips. The confluence of middle and left hepatic venous and the vena cava is exposed medial to lateral with cold sharp dissection.
Depending on whether a conventional or anterior approach is chosen, the right triangular and coronary ligaments can be divided before or after the parenchymal transection, respectively, taking advantage of the lateral position of the patient.
The occlusion of hepatic vascular inflow and outflow is not required for lesions ≤3 cm in diameter or when performing resection of the left lateral lobe. However, the hepatic vascular inflow and outflow must be occluded when removing lesions >5 cm in diameter or when performing anatomic liver resection.
If hepatic pedicle occlusion is anticipated, the Pringle maneuver, probably the simplest method of inflow limitation, currently can be achieved laparoscopically. Although total vascular inflow occlusion can be easily performed, ischemic reperfusion injuries can lead to increased postoperative morbidity. On the other hand, hemihepatic inflow occlusion, leading to hemihepatic ischemia, decreases the amount of liver parenchyma under reperfusion damage and offers the advantage of reduced blood loss. Half-Pringle maneuver is feasible and safe and may be achieved by the advanced armamentarium in laparoscopic right and left hepatectomy [55].
On the basis of the laparoscopic ultrasonography or demarcation lines induced by interruption of hepatic artery and portal vein flow, the transection plane is outlined on the liver capsule with monopolar electrocoagulation.
24.6.3 Parenchymal Resection
The main technical challenge of laparoscopic liver resection remains to be hemorrhage during major anatomic parenchymal dissection, especially in cirrhotic patients. The choice of technique for resection is therefore important. According to our experience, multiple instruments are needed, including ultrasonic coagulation scalpel, bipolar electrocoagulation, Hem-o-lok clips, and endoscopic linear cutter staplers (Echelon 60 mm). The hepatic capsule and the superficial 2–3 cm of parenchyma can be dissected by Harmonic ultrasonic scalpel. Vessels and biliary ducts less than 3 mm in diameter encountered during the dissection of the superficial and deep parenchyma can be ligated and transected using Harmonic ultrasonic scalpel or bipolar electrocoagulation. Larger arteries and bile ducts are ligated using Hem-o-lok. Endoscopic linear cutter stapler (Echelon 60 mm) is used for Glisson’s pedicles, portal branches, and hepatic veins. The argon beam coagulator is primarily applied for the parenchyma resection margin hemostasis.
24.6.4 Specimen Extraction
In all cases, the specimen is placed in a plastic bag and extracted, and we suggest it be extracted through an enlarged port site. Except the benign lesions, fragmentation of specimen must be avoided to allow proper pathological evaluation.
24.7 Surgical Procedures
24.7.1 Laparoscopic Limited Resection
Recent data suggest that wedge resection is adequate for a benign tumor, a metastasis tumor, or a solitary and small malignant primary tumor in the liver [56–58]. Superficial lesions in segments 2–6 are the best indication to this procedure, which do not need dissection of the first and second porta hepatis.
The patient was placed in supine position; the surgeons stood on the left or right side of the patient, according to the lesion site. Four ports are necessary in this procedure. After the pneumoperitoneum, placement of trocar and the necessary free of the relative ligament were all finished, the margin of parenchymal dissection was marked 1–2 cm away from the tumor using electrocoagulation and subsequently the capsule was cut.
The operational field should be kept clean and clear. For the limited resection of tumor with a large size, the area of the liver wound was usually large, and it would be hard to coagulate because it was impossible to block the inflow and outflow of blood as was done in regular LLR. To solve this problem, the primary feeding vessel could be reconstructed using contrast-enhanced CT or MRI preoperatively and before the resection of tumor, ligated. The incisional surface was covered with hemorrhage materials optionally, and whether to place a drainage tube should depend on the area of incisional surface and the condition of coagulation.
If possible, laparoscopic ultrasonography was used to help localize the tumors, demarcation of vascular structures, satellite nodules (if any), and an adequate tumor-free margin. The surgeon must be able to visualize the lesion on cross-sectional imaging if no laparoscopic ultrasound probe is available and confirm that a safe margin can be obtained without damaging the pedicles or encountering large hepatic vein branch.
For malignant lesions, 10–20 mm margins are measured using ultrasonography and marked using electrocautery. For benign lesions, a wide margin is not required. Parenchymal dissection is performed with the Harmonic scalpel and follows the marked margins. Hemostasis is achieved by bipolar electrocoagulation or laparoscopic argon beam coagulator. As larger vessels are encountered, clips should be applied. Maintaining a bloodless field is critical and can only be accomplished by constant irrigation of the dissection area. If significant veins, ducts, or segmental pedicles are encountered, a segmentectomy should be conducted to prevent necrosis or biliary fistula. The specimen was placed inside a large plastic bag and subsequently removed through the enlarged trocar port. For large cases, the specimen can be extracted by connecting two ports. Although suprapubic incision is usually used abroad, which is more hidden, the procedure is too tedious.
Laparoscopic limited resection for hepatic cellular carcinoma in the borderline of segments VIb and V
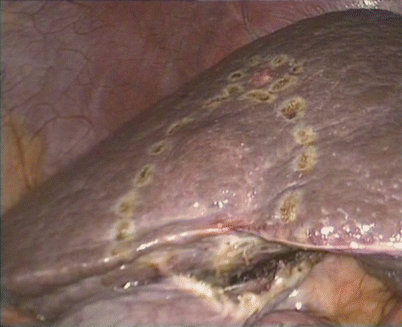
Fig. 24.12
The margins were marked using electrocautery
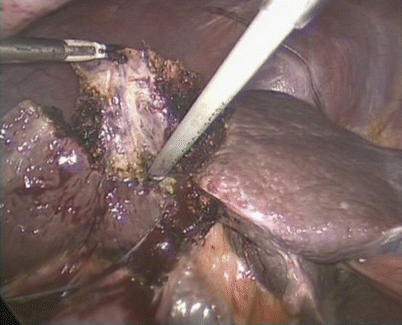
Fig. 24.13
The Harmonic scalpel and BiClamp were combined for transection of the liver
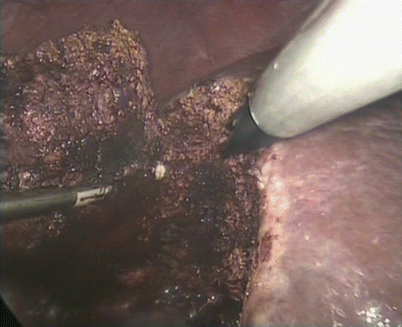
Fig. 24.14
The feeding vessel was clipped using absorbable clips
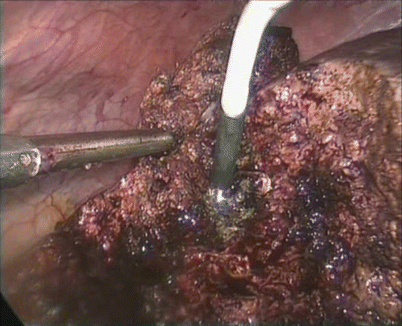
Fig. 24.15
Coagulation was achieved using argon beam coagulator
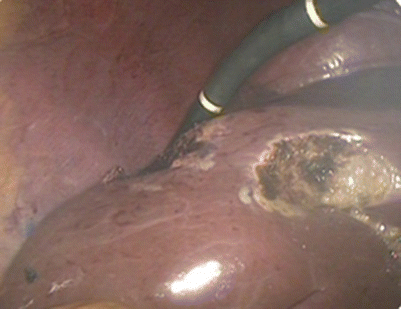
Fig. 24.16
Examination using laparoscopic ultrasound
Laparoscopic limited resection for hepatic cellular carcinoma in segment VI
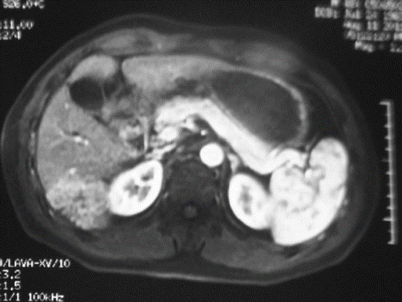
Fig. 24.17
Hepatic cellular carcinoma in segment VI
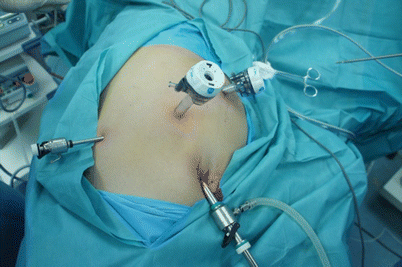
Fig. 24.18
The placement of trocar
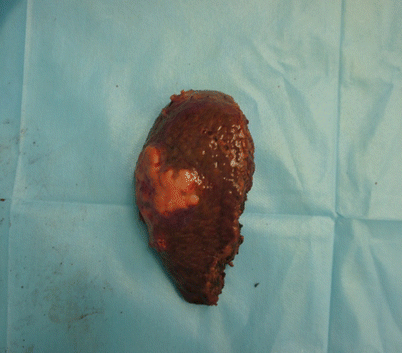
Fig. 24.19
Specimen
24.7.2 Laparoscopic Segmentectomy
Anatomic segmentectomy plays an important role in maximizing the postoperative liver function reserve. Besides, the oncological advantage of anatomic segmentectomy in eradicating potential intrahepatic metastases has been clearly shown in the surgical management of hepatocellular carcinoma. Therefore, laparoscopic segmentectomy that minimizes the loss of normal hepatic parenchyma while ensuring adequate oncological margins has been developed and performed for segments I to VIII [59].
Rather than reliance on surface anatomy, ultrasonography is used to determine Couinaud segmental anatomy and then mark on the liver’s surface. Bilateral traction maintained by the assistance of atraumatic graspers allows the parenchymal transection plane to be seen clearly by the operating surgeon. During the parenchymal dissection, a combination of ultrasonic scalpel and bipolar electrocoagulation is used. Section pedicles are ligated with locking clips, and other vessels or ducts are ligated with metal clips as they are encountered. Additional hemostasis is obtained by using bipolar electrocoagulation and the argon beam coagulator. Drains are used only if there is concern about intraoperative injury of the biliary duct or the adequacy of hemostasis.
24.7.3 Laparoscopic Left Lateral Sectionectomy
Because of the favorable anatomy of the hepatic left lateral section, its resection was the first formal liver resection reported using the laparoscopic approach [60], which was also generally accepted as the introduction of LLR [17, 29–31, 33, 45, 61, 62].
We have summarized the protocol of laparoscopic left lateral sectionectomy as a modeling method [26, 38], and a number of case series proved the feasibility and safety. Currently, laparoscopic left lateral sectionectomy has been considered the most suitable anatomical resection for the laparoscopic approach and the standard surgery for lesions in segments II and III, and the technical details are as follows.
The patient was placed in a supine position with the surgeon and the assistant standing on the patient’s right side and the scope handler on the left. The patients were usually held with head higher than the foot and the right side higher than the left. The monitor was placed in the head side of the patient, deviated to the left. Pneumoperitoneum is created by infusion of carbon dioxide under the umbilicus, and the other three trocars were placed as discussed above.
The ligamentum teres hepatis, the falciform ligament, the left coronary ligament, and the left triangular ligament were successively divided using the ultrasonic scalpel. The left lobe was lifted by the assistant to divide the lesser omentum to the base of the venous ligament (for some patients, the division of the lesser omentum is not necessary). Enough division is extremely important to the following surgery, and there is no need to expose the left hepatic vein or the inferior vena cava.
The liver parenchyma in the anterior, superior, and inferior of the vascular pedicle of the segment II/III was cut moderately, resulting a rough dissection of segmental pedicle. An endoscopic linear cutter stapler was placed through the right trocar and cut the segmental pedicle as well as nearby liver parenchyma. EC60 with golden nail box, Echelon, was suggested for the previous operation.
Transaction of the liver then continued, and the parenchyma in the anterior, superior, and inferior of left hepatic vein was slightly cut, resulting a rough dissection of the left hepatic vein, which should be able to be cut entirely by endoscopic linear cutter stapler. Then the left hepatic vein as well as nearby liver parenchyma was cut. To make sure that the left hepatic vein is entirely ligated, before the cut of the tissue, the assistant should put a grasper through the left trocar and grasp the left triangular ligament, pulling the left lateral section ventralward and downward and exposing the superior tip and the anterior tip at the same time. When the left hepatic vein was not ligated sufficiently, an absorbable clip could be used to clip the remnant tissue. Usually, the extrahepatic part of the left hepatic vein is not necessarily to be dissected.
The incisional surface and the hepatic stump were inspected for any bleeding and bile leak. Homeostasis was obtained using BiClamp for active bleeding points, and clips or Prolene sutures were used for any bile leak. A drain tube should be placed through the right trocar. The specimen is removed in an impermeable bag through an extended trocar.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







