Laparoscopic Colon and Rectal Surgery
Roberto C. M. Bergamaschi
Sergio W. Larach
Alessio Pigazzi
Slawomir Marecik
Elsa B. Valsdottir
Salim Amrani
A little learning is a dangerous thing.
—ALEXANDER POPE (1688-1744), Essay on Criticism, Part ii
A learning curve is not a valid justification for patient injury.
—State of New York Department of Health Memorandum—Series 92-20, June 12, 1992
▶ HISTORY
Laparoscopic colorectal surgery has evolved considerably, especially over the past 20 years. In 1991, Schlinkert193 described the surgical technique of laparoscopic-assisted right hemicolectomy for cancer in a single case report. A lateralto-medial dissection was performed with intracorporeal vessel ligation through the use of five ports. A horizontal laparotomy with division of the right rectus muscle was carried out to resect, anastomose, and retrieve the specimen. In 1991, Fowler and White75 described the surgical technique of laparoscopic-assisted sigmoid resection with intracorporeal vessel ligation, also in a case report. A muscle-splitting laparotomy was carried out in the left lower abdominal quadrant in order to resect the bowel and to fashion a purse-string suture around the circular stapler’s anvil. The suture was placed in the proximal end of the descending colon. Also in 1991, Jacobs and colleagues101 reported a case series of 20 patients who underwent laparoscopic-assisted right hemicolectomy or sigmoid resection for benign and malignant diseases.
Following these reports, colorectal surgeons in Germany and the United States undertook laboratory investigations. Kockerling and associates80,116 performed an experimental surgery using intracorporeal anastomotic techniques in large animals and developed a reusable laparoscopic purse-string suture instrument (Figure 19-1). Cohen and coworkers49 presented the initial experimental results in pigs with intracorporeal anastomoses and with mesenteric closure with the use of staples. Böhm and colleagues34 performed an oncologic right colectomy with intracorporeal ileocolic anastomosis in a canine model.
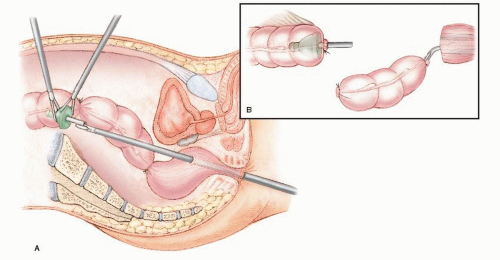
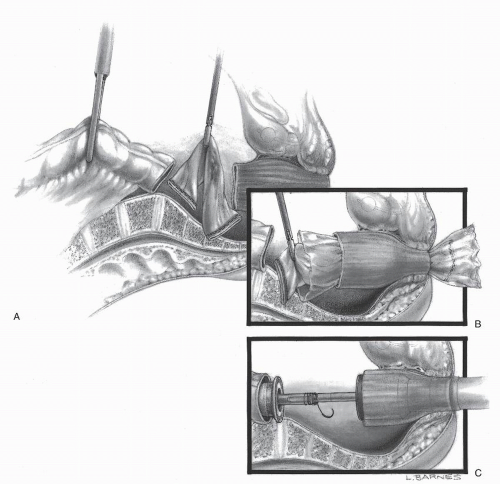
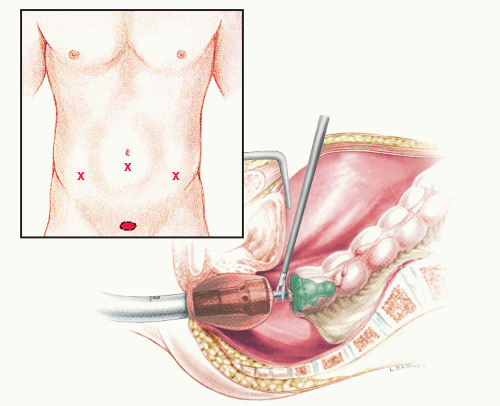
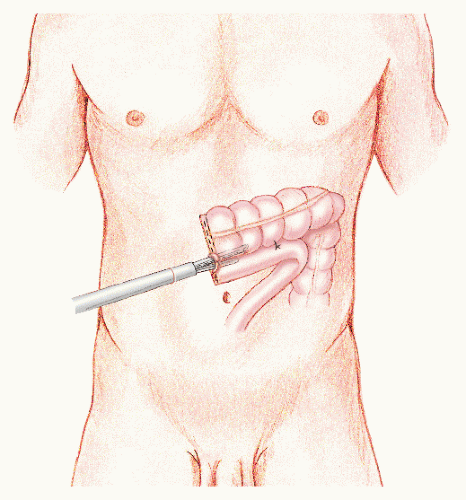
In 1992, Phillips and associates166 published a series of 51 patients who underwent a variety of colorectal resections with a laparoscopic, intracorporeal, hand-sewn purse-string suture and transanal natural orifice specimen extraction (NOSE). In 1993, Bleday and coworkers33 described a similar intracorporeal technique using a colonoscopic snare for transanal NOSE (Figure 19-2). In 1995, Mentges and associates described a technique that involved a proctotomy in the anterior rectal wall in order to insert the anvil of a circular stapler into the peritoneal cavity and to perform a NOSE by means of transanal endoscopic microsurgery (TEM) (Figure 19-3).142 Also in 1994, Darzi and colleagues described a left colon resection with transanal NOSE followed by a triple-stapled intracorporeal colorectal anastomosis (Figure19-4).55 In 2000, the use of a 33-mm port (Figure 19-5), which allowed the insertion of the anvil of a circular stapler into the abdominal cavity, was described to circumvent the requirement to use transanal NOSE for this purpose (Figure 19-6).20
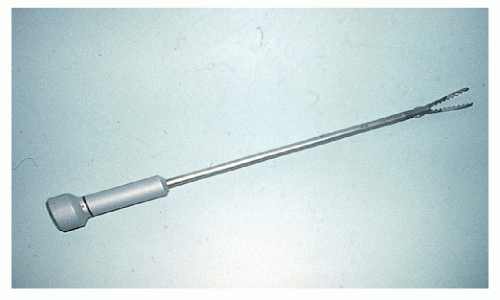 FIGURE 19-1. Reusable laparoscopic purse-string suture device. (Courtesy of Professor Ferdinand Kockerling.) |
Most of the aforementioned intracorporeal techniques have not stood the test of time. In fact, nonrandomized and randomized data have established similar outcomes following intracorporeal and laparoscopic-assisted sigmoid colectomy.28,29 However, attention has been drawn to the specimen extraction site. The traditional lower abdominal quadrant muscle-splitting incision for performing left colectomy was being challenged. For example, a Pfannenstiel incision affords direct visualization of the colorectal anastomosis fashioned at the level of the sacral promontory, thus obviating the requirement for reestablishing a pneumoperitoneum prior to performing the anastomosis (Figure 19-7).17 In 2005, Chang and coworkers popularized hand-assisted laparoscopic sigmoid colectomy using this incision.41
 FIGURE 19-2. A: Colonoscopic snare transanal retrieval of specimen. B: Laparoscopic double-stapled colorectal anastomosis. |
At first glance, one might interpret that the earlier literature seemed to be focused on technical feasibility. However, as early as 1992, Wexner and colleagues reported no immediately recognizable benefits for patients undergoing laparoscopic total colectomy, shifting the focus to that of clinical outcomes.221 That same year, Pezet and coworkers warned about seeding the abdominal wall during laparoscopic cholecystectomy for gallbladder cancer.165 In 1993, their report was echoed by a number of case reports of incisional recurrences after laparoscopic surgery for colon cancer.5,77,160,219 Although the 1994 American Society of Colon and Rectal Surgeons registry data published an incisional recurrence rate of 0.4% after laparoscopic surgery for colon cancer,174 the aforementioned anecdotal reports generated widespread concern. Colorectal surgeons in Europe and in the United States went back to the laboratory generating at least 72 articles on animal studies, the validity of which has been questioned.15 In Norway, the consensus was not to attempt laparoscopic surgery for cure of colon cancer prior to level 1 evidence becoming available.25 Eventually, the results of three adequately powered, randomized controlled trials reported no significant differences in oncologic outcomes at 3- and 5-year followup.88,154,217 Although all three randomized controlled trials did not include transverse colon cancer, the Medical Research Council conventional versus laparoscopic-assisted surgery in colorectal cancer (CLASICC) trial did incorporate patients with rectal cancer and concluded that routine use of laparoscopic surgery for rectal cancer is not justified.88,103 As of this
writing, at least two randomized controlled trials are evaluating laparoscopic surgery for rectal cancer.23
writing, at least two randomized controlled trials are evaluating laparoscopic surgery for rectal cancer.23
The 20th century saw the development of an organized approach to performing laparoscopic colorectal surgery. For example, Senagore pioneered standardization of surgical technique, introducing sequential operative steps for accomplishing sigmoid colectomy.197 Evolution of laparoscopic right colectomy resulted initially in performing 5 operative steps,231 then 8,196 and ultimately 10.27 “Hindered” or facilitated, gasless or with gas, extracorporeal or intracorporeal, multiple- or single-port laparoscopic colon and rectal surgery is still evolving, with many more authors and players entering the fray.
▶ INTRODUCTION
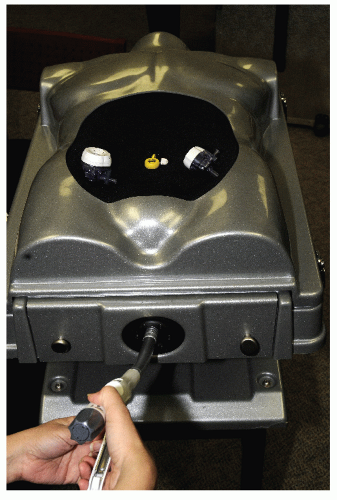
Twenty years have elapsed since the first reports on laparoscopic colectomy appeared in the literature.75,101,193 Yet, readers might be surprised to know that there has not been a consistent use of a defined terminology in the literature. When in its infancy, there was understandable confusion regarding the nomenclature, mostly reflecting its evolution. The term laparoscopic facilitated,180 although it did not gain popularity, described exteriorizing the colon through a limited laparotomy following lateral-to-medial mobilization. The term laparoscopic-assisted, however, appeared frequently in the literature, indicating laparoscopic vessel ligation with medial-to-lateral mobilization prior to exteriorizing the bowel for extracorporeal (for lack of a better term) resection and anastomosis. Intracorporeal referred to the adoption of laparoscopic bowel transection and laparoscopic anastomosis with specimen retrieval in a plastic bag. Even the term minimally invasive (which could function as an umbrella term, encompassing all such concepts) has been challenged by the supporters of the phrase minimal access surgery. It is now recognized that defined terminology is a prerequisite for allowing a meaningful flow of information for any nascent field to develop and grow. For example, NOTES is an acronym for natural orifice transluminal endoscopic surgery, which is actually endoscopic surgery, an established concept that does not include skin incisions.2 Curiously, the one concept that seems intuitive, and one that would not need an elaborate definition, has generated a publication dedicated to define surgery performed through a single port (laparoendoscopic single site [LESS]).83 Currently, LESS colectomy is considered a “boutique procedure,” a niche, or specialized application (from French for “shop,” via Latin from Greek 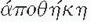 [apothēkē], “storehouse”).134 On the subject of etymology, the definition of a trocar (variant: trochar; from French: alteration of trois-carré from trois [three] + carré [square]—i.e., “three-edged,” triangular) and its evolution is an interesting one. The term initially identified a cannula several millimeters in diameter. Later, it was broadened to include trocars several centimeters in diameter. Needlescopic surgery performed using instruments with a diameter of 2 mm did not represent an option for conducting minimally invasive colorectal surgery because of technical limitations of the optics. Other concerns include improper illumination, inadequate resolution, and lack of clarity, in addition to problems associated with instrument sturdiness and manipulability. An example of a larger port is that of a 33 mm.20 This size permits insertion of the anvil of a circular stapler into the abdominal cavity. A 12-cm port allows insertion of the surgeon’s hand. Interestingly, these two large ports reflect opposite visions. The former is the intracorporeal vision with the unresolved issue of specimen extraction.
[apothēkē], “storehouse”).134 On the subject of etymology, the definition of a trocar (variant: trochar; from French: alteration of trois-carré from trois [three] + carré [square]—i.e., “three-edged,” triangular) and its evolution is an interesting one. The term initially identified a cannula several millimeters in diameter. Later, it was broadened to include trocars several centimeters in diameter. Needlescopic surgery performed using instruments with a diameter of 2 mm did not represent an option for conducting minimally invasive colorectal surgery because of technical limitations of the optics. Other concerns include improper illumination, inadequate resolution, and lack of clarity, in addition to problems associated with instrument sturdiness and manipulability. An example of a larger port is that of a 33 mm.20 This size permits insertion of the anvil of a circular stapler into the abdominal cavity. A 12-cm port allows insertion of the surgeon’s hand. Interestingly, these two large ports reflect opposite visions. The former is the intracorporeal vision with the unresolved issue of specimen extraction.
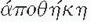 [apothēkē], “storehouse”).134 On the subject of etymology, the definition of a trocar (variant: trochar; from French: alteration of trois-carré from trois [three] + carré [square]—i.e., “three-edged,” triangular) and its evolution is an interesting one. The term initially identified a cannula several millimeters in diameter. Later, it was broadened to include trocars several centimeters in diameter. Needlescopic surgery performed using instruments with a diameter of 2 mm did not represent an option for conducting minimally invasive colorectal surgery because of technical limitations of the optics. Other concerns include improper illumination, inadequate resolution, and lack of clarity, in addition to problems associated with instrument sturdiness and manipulability. An example of a larger port is that of a 33 mm.20 This size permits insertion of the anvil of a circular stapler into the abdominal cavity. A 12-cm port allows insertion of the surgeon’s hand. Interestingly, these two large ports reflect opposite visions. The former is the intracorporeal vision with the unresolved issue of specimen extraction.
[apothēkē], “storehouse”).134 On the subject of etymology, the definition of a trocar (variant: trochar; from French: alteration of trois-carré from trois [three] + carré [square]—i.e., “three-edged,” triangular) and its evolution is an interesting one. The term initially identified a cannula several millimeters in diameter. Later, it was broadened to include trocars several centimeters in diameter. Needlescopic surgery performed using instruments with a diameter of 2 mm did not represent an option for conducting minimally invasive colorectal surgery because of technical limitations of the optics. Other concerns include improper illumination, inadequate resolution, and lack of clarity, in addition to problems associated with instrument sturdiness and manipulability. An example of a larger port is that of a 33 mm.20 This size permits insertion of the anvil of a circular stapler into the abdominal cavity. A 12-cm port allows insertion of the surgeon’s hand. Interestingly, these two large ports reflect opposite visions. The former is the intracorporeal vision with the unresolved issue of specimen extraction.Colorectal anastomosis following laparoscopic left colon resection differs from its right-sided counterpart because the rectum cannot be exteriorized. Following proximal colon transection, a purse-string suture of the proximal colon end may be hand-sewn extracorporeally. The laparoscopic, intracorporeal, hand-sewn purse-string suture, although feasible, has not become popular among colorectal surgeons, mainly because of lack of training in laparoscopic suturing. The advent of robotic technology will most likely have an impact on implementation of intracorporeal suturing with its requisite intracorporeal knot-tying. In fact, the robot has given back to surgeons the two degrees of freedom lost by the use of laparoscopic, nonwristed instrumentation. Nevertheless, the fact remains that safe specimen extraction still requires at least the enlargement of a port site incision unless transvaginal NOSE is considered in female patients.162 As mentioned, the port allowing insertion of the surgeon’s hand reflects a different vision for accomplishing laparoscopic surgery. However, hand-assisted laparoscopic surgery (HALS) does provide reasonable solutions to some legitimate concerns. For example, HALS allows manual palpation of a rectal tumor for providing adequate distal transection of the rectum as well as decreased operating time for laparoscopic total colectomy by expediting transverse colon mobilization and safe vessel ligation.130
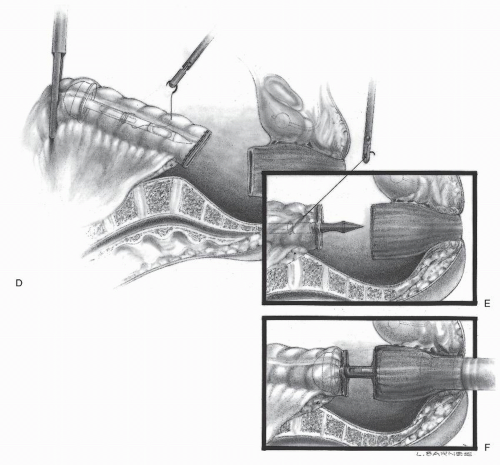 FIGURE 19-4. (continued) D: The anvil is placed into the proximal colon, and the circular stapler is withdrawn from the anus. The suture is passed from within the bowel lumen to provide a “handle.” The proximal bowel is stapled. E: The anvil is controlled by means of the suture and brought through the staple line. The staple line is then cut. F: The rectum is then stapled across and the circular stapler inserted in accordance with the standard, “double-stapling” technique (see Chapter 24). |
This chapter represents current opinion on the impact of laparoscopic colon and rectal surgery performed with
pneumoperitoneum through multiple ports on several diseases of the colon and rectum. Herein, however, no attempt is made to provide a systematic review of the evidence available in the literature because the objective is to present an insight into laparoscopic surgical techniques, the authors’ personal opinions, and an overview of the literature dealing with this subject.
pneumoperitoneum through multiple ports on several diseases of the colon and rectum. Herein, however, no attempt is made to provide a systematic review of the evidence available in the literature because the objective is to present an insight into laparoscopic surgical techniques, the authors’ personal opinions, and an overview of the literature dealing with this subject.
▶ LEARNING
The subject of learning the technique for performing any surgery, especially that of laparoscopic colorectal surgery, is of paramount concern and importance. A learning curve is defined as a graphical description of the rate of learning.181 Initially introduced in educational psychology, these two words have acquired considerable prominence and have been used extensively in the laparoscopic colorectal surgery literature. Classically, it is noteworthy that characterizing a learning curve as “steep” indicates that proficiency can be attained over a short experience.175 Unfortunately, the phrase is a misnomer—the laparoscopic colorectal surgery literature refers to a steep learning curve as indicative of the opposite—that is, a rather long time for acquiring the skill.
Goodness of Fit Metrics
The goodness of fit of a given model describes how the model fits a set of data.194 The term, goodness of fit, is used here as an analogy to characterize the complexity of evaluating the learning curve in laparoscopic colorectal surgery. The metrics used in the literature to appraise the learning curve include operating time, conversion rates, and complication rates. However, readmission rates should probably be added. A study involving chronologic groups reported a decrease in operating time from 250 to 156 minutes over the first 75 cases, following which operating time remained stagnant.227 Another study observed that surgeons were getting faster despite the increasing complexities of caseloads, suggesting improved skills.1 Dinçler and associates suggested shifting the focus from solely decreased operating time to a multidimensional analysis, which would take into account the conversion and complication rates as well.60 Chen and colleagues came to the conclusion that operating time seemed to be a poor surrogate for evaluating the learning curve.43 Thus, the question becomes, Do we need to get faster?227
 FIGURE 19-6. Insertion of anvil through 33-mm suprapubic port and intracorporeal hand-sewn purse-string suture. |
Conversion to open surgery has also been viewed as a metric for evaluating the learning curve. There are, however, a number of other factors impacting on conversion rates, such as a patient’s American Society of Anesthesiologist (ASA) and body mass index (BMI) scores; the type of resection; operative findings, such as the presence of an internal fistula and/or an intra-abdominal abscess; and more.206 The complication rate is probably the single most important metric. Bennet and coworkers reported a significant decrease in postoperative complication rates after one performs 40 operations.16 Reissman and colleagues showed that complication rates were significantly higher after total colectomy (42%) as compared with segmental colectomy (19%) and nonresectional procedures (12%).176
So what is the magic number? Several studies have been published in an attempt to identify that figure. Although Simons and associates suggested 11 to 15 procedures in
1995,201 that same year Wishner and colleagues increased that number to 35 to 50.227 A decade later, Tekkis and coworkers estimated 55 right colectomies and 62 left colectomies to be the “right numbers.”207 Interestingly, both COST (Clinical Outcomes of Surgical Therapy) and COLOR (Colon Cancer Laparoscopic or Open Resection) trials required participating surgeons to perform 20 laparoscopic colectomies before entering patients in the trials. In conclusion, there is truly a “poorness of fit” in comparing learning models to the literature data with respect to the learning curve in laparoscopic colorectal surgery.
1995,201 that same year Wishner and colleagues increased that number to 35 to 50.227 A decade later, Tekkis and coworkers estimated 55 right colectomies and 62 left colectomies to be the “right numbers.”207 Interestingly, both COST (Clinical Outcomes of Surgical Therapy) and COLOR (Colon Cancer Laparoscopic or Open Resection) trials required participating surgeons to perform 20 laparoscopic colectomies before entering patients in the trials. In conclusion, there is truly a “poorness of fit” in comparing learning models to the literature data with respect to the learning curve in laparoscopic colorectal surgery.
Simulation
Psychomotor Skills
Surgical training remains to a great extent, even today, mired in William Halsted’s century-old apprenticeship model.90 Although in the past the Halstedian method has produced generations of fine technical surgeons, its adequacy for training future laparoscopic surgeons is questionable. Among a number of possible factors, such as the problems associated with the fulcrum effect, the loss of tactile sensation, increased complexity of the techniques, less independence of the residents, shorter work hours, the most important factor probably is the disruption of the constrained coupling between the surgeon’s hands and eyes through the interposition of a video camera, moving independently. Thus, although cut, sew, and tie remain the staples of one’s surgical craft, laparoscopic surgery requires the development of a new skill set. Several factors including random educational opportunities, concern for the patient, time constraints, surreptitious dissection by the attending surgeon or faculty, distractions, interpersonal issues, and cost, among others, suggest that the operating room (OR) is a suboptimal classroom to obtain a proper learning experience, at least initially.
Training outside of the OR offers a structured educational opportunity as well as stress modulation (reduction of a resident’s stress through procedures undertaken in a simulation laboratory). Although isoperformance is a well-documented teaching principle for flight simulation, its introduction into education for laparoscopic surgery raises the issue of transferring skills acquired in simulation models to the OR. A systematic review of randomized and nonrandomized data by the Royal Australasian College of Surgeons was inconclusive as to what extent skills learned during simulation in laparoscopic surgery are transferable to the OR.204 Inanimate simulation models would seem to be an appropriate method for implementing the acquisition of psychomotor skills. In fact, it is the dynamic coupling of hands, eyes, and the interposed camera (rather than the static graphic display) that allows skill acquisition. Inanimate physical rather than a virtual simulator should be used because the latter does not include an independently moving camera nor does it feature any tactile feedback.104
Learning motor skills requires more than simply passive observation.21 That is why one should probably dismiss the practice of mastering skills in minimally invasive surgery through a camera holder’s role. In fact, robots will increasingly hold the telescope and allow the surgeon to steer an otherwise independently moving camera.212
 FIGURE 19-8. A: Path length, the measurement in millimeters of the movement of the laparoscopic instrument tips during simulation, as used by a novice; (B) as used by an expert. |
The process of acquisition of motor skills may be broken down into three phases.108 Both stand-up and CD-ROM tutorials are adequate for task understanding (phase 1). The interaction between task practicing (phase 2) and feedback is essential to skill acquisition. (For phase 3, see Procedural Skills.) Novices compare their own performance to that of CD-ROM to minimize the difference (internal feedback). Experts (external feedback) provide learners with information about the procedure’s effectiveness and quality of the operative end product. The former can be evaluated by objective assessment of a number of outcome measures (goal- and nongoal-directed actions, forces and torques, and operating time), and the latter can be assessed by end product analysis (accuracy error, tissue damage, water tightness, etc.). The reported poor correlation between the two may indicate that a range of pattern of movements may result in a similar quality of end product. A number of advantages (construct validity, time and cost efficiency, resident autonomy in performance, and reduced examiner bias) suggest that product analysis is suited for skill assessment despite its low reliability. There is no consensus on which dexterity drills should be incorporated into simulation models for the acquisition of motor skills.18 Reliability and validity (content, construct, criterion, concurrent, and predictive) of current simulation models have been evaluated only to a limited extent.54
Metrics
A fundamental issue for training with simulators has been the need for metrics.52 Examples of previously validated content-valid metrics include accuracy error, knot slippage, leakage, operating time, and tissue damage.213 Hybrid simulators (combination of real instruments and box trainer with computer monitoring) offer software-generated metrics not available in the operating room, such as path length (Figure 19-8) and smoothness of laparoscopic instrument movement. However, studies have shown no correlation between simulator-generated metrics and content-valid metrics except for operating time.40 Most simulation studies compare metrics at baseline testing with metrics at unmentored final testing. This is the definition of responsiveness, which is a change in performance over time.95,207 Nonetheless, additional challenges to the validity of any simulation metrics derive from the so-called ceiling effect: learning the drill without acquiring the skill.
Procedural Skills
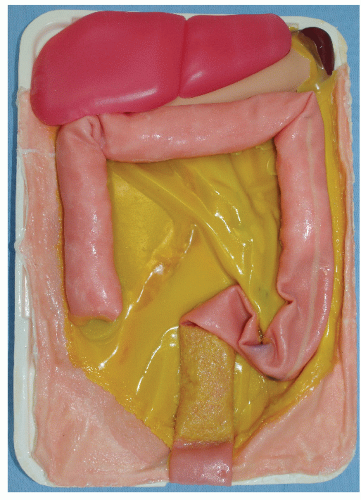
A stepwise draft for learning outside of the OR involves the acquisition of procedural skills without distinct cognitive awareness (phase 3). Learning the setup and exposure for a surgical procedure requires simulation models, such as anatomical. Ethical issues, limited availability, and high costs have limited the use of live animal or fresh human cadaver models. An alternative bench model consists of en bloc resected fresh animal organs placed in a simulator and artificially perfused. Whether this model has the potential for transferring skills to the OR remains to be proven, particularly in the specific case of colorectal surgery. Currently, hybrid simulators specially designed for laparoscopic colorectal surgery feature an anal opening (Figure 19-9) and include a disposable abdominal tray (Figure 19-10). Unfortunately, the human anatomy associated with such trays is questionably accurate and valid and is costly. Nevertheless, simulation studies with laparoscopic sigmoidectomy have reported good responsiveness among surgery residents, with significantly decreased operating time and lower anastomotic leak rates.70
Recently, task analysis of skills involved in laparoscopic colorectal surgery has been performed according to the Likert scale* and the Delphi process†188 When responding to a Likert questionnaire item, individuals specify their level of agreement or disagreement on a symmetric agree-disagree
scale for a series of statements. Delphi is based on the principle that decisions from a structured group of individuals are more accurate than those from unstructured groups. The experts answer questionnaires in two or more rounds. After each round, a facilitator provides an anonymous summary of the experts’ decisions from the previous round as well as the reasons they provided for their judgments. Thus, experts are encouraged to revise their earlier answers in light of the replies of other members of their panel. Simulation research is an educational challenge that requires a change of attitude in the surgical community in addition to funding.178 The primary end point of educational research studies should be to provide evidence that proper instruction has an impact on the quality of patient care.
scale for a series of statements. Delphi is based on the principle that decisions from a structured group of individuals are more accurate than those from unstructured groups. The experts answer questionnaires in two or more rounds. After each round, a facilitator provides an anonymous summary of the experts’ decisions from the previous round as well as the reasons they provided for their judgments. Thus, experts are encouraged to revise their earlier answers in light of the replies of other members of their panel. Simulation research is an educational challenge that requires a change of attitude in the surgical community in addition to funding.178 The primary end point of educational research studies should be to provide evidence that proper instruction has an impact on the quality of patient care.
▶ OPERATING ROOM
The First Port
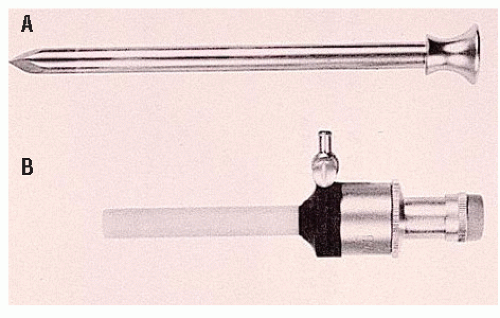
Safe access to the abdominal cavity is the initial critical step during surgery. Although surgeons may have a personal preference for the open or the blind (Veress) technique, the fact remains that many of the access-related complications are due to the first trocar insertion. The Hasson technique was initially described as an alternative for accessing the abdominal cavity in previously operated patients (Figure 19-11).93 By choosing a cutdown approach, the surgeon can introduce a blunt tip trocar into the abdominal cavity under direct visualization, as opposed to a blind entry with the Veress needle. In fact, most surgeons favor a cutdown technique for reoperative surgery.
When dealing with a reoperation, it is advisable to avoid scars because the risk of inadvertent injury to intraabdominal organs is increased. Assuming that the patient will undergo a midline laparotomy, the most frequently encountered scenario, the initial trocar should be placed
lateral to the rectus muscle sheath in the midpoint between the lower edge of the rib cage and the anterior superior iliac spine along the anterior axillary line. The surgeon must also be careful not to place the trocar too far laterally because of the risk of injuring the descending colon. Detailed awareness of the patient’s prior abdominal operations will, of course, assist in determining which side of the abdomen the first trocar should be placed. For example, if the patient is to undergo a left colectomy, the first trocar should be inserted on the right side of the abdomen. The opposite concept is true for surgeries performed on the right side of the patient. Optical access trocar systems have been developed, which allow the surgeon to visualize the abdominal wall layers as the trocar enters the peritoneal cavity. These ports are made with the intention of minimizing organ injury while gaining laparoscopic access. There are currently two optical view trocars: one of them uses a blade and the other uses a rotating, sharp, plastic, clear tip, both under direct laparoscopic view. Regardless of what type of trocar the surgeon chooses, the insertion technique should involve minimal force and consistent direct visualization.
lateral to the rectus muscle sheath in the midpoint between the lower edge of the rib cage and the anterior superior iliac spine along the anterior axillary line. The surgeon must also be careful not to place the trocar too far laterally because of the risk of injuring the descending colon. Detailed awareness of the patient’s prior abdominal operations will, of course, assist in determining which side of the abdomen the first trocar should be placed. For example, if the patient is to undergo a left colectomy, the first trocar should be inserted on the right side of the abdomen. The opposite concept is true for surgeries performed on the right side of the patient. Optical access trocar systems have been developed, which allow the surgeon to visualize the abdominal wall layers as the trocar enters the peritoneal cavity. These ports are made with the intention of minimizing organ injury while gaining laparoscopic access. There are currently two optical view trocars: one of them uses a blade and the other uses a rotating, sharp, plastic, clear tip, both under direct laparoscopic view. Regardless of what type of trocar the surgeon chooses, the insertion technique should involve minimal force and consistent direct visualization.
Vascular Injuries
Although the Hasson technique will not completely eliminate the risk for major vascular injuries, there is evidence that suggests this risk is decreased. Although vascular injuries are rare, they must be recognized and repaired by means of an immediate laparotomy. Prior to converting, the surgeon may attempt to control the bleeding vessel with a laparoscopic Satinsky clamp. Much of the evidence related to vascular injuries secondary to laparoscopic trocar insertion comes from case reports. In a review of 629 trocar incident reports, 408 cases were associated with major vascular injuries, resulting in 26 deaths. The vessels most commonly injured were the aorta (23%) and the vena cava (15%).32 Optical view ports had been used, which indicates that such accessories cannot completely prevent trocar insertion-related injuries.
Bowel Injuries
Unfortunately, neither the rate of bowel injuries nor related death rates have been reduced by the Hasson technique. It is worth repeating that the Hasson approach should be employed at a site away from preexisting scars. In fact, 40% of bowel injuries have been reported to be due to insertion of additional trocars. Therefore, insertion of ports must be performed under direct vision with sufficient intraperitoneal space. This entails adequate relaxation of the abdominal wall musculature by the anesthesiologist.
Pneumoperitoneum
There is no question about the need for adequate pneumoperitoneum. Surgeons should have a thought process that evaluates all possible causes of insufficient pneumoperitoneum. Once all common causes are ruled out, the surgeon should communicate with the anesthesiologist to ensure that proper paralysis of the anterior abdominal wall has been provided. It is beneficial for patients to be operated on with the lowest possible intra-abdominal pressure. In fact, there is very little difference in the exposure provided with 10 versus 15 mm Hg.157 If the operation is performed mostly in the pelvis, it may be sufficient to operate with an intra-abdominal pressure of less than 10 mm Hg because the pelvis is a bony structure.
The surgeon must also take into account the patient’s body habitus when inducing and maintaining pneumoperitoneum. An abdominal wall thickness of 10 cm or more will require a 15-cm long port, rather than the ordinary 10-cm length. If the trocar is too short for the abdominal wall thickness, insufflation of CO2 will lead to subcutaneous emphysema with subsequent increase in tidal volume. Certain medical comorbidities such as chronic obstructive pulmonary disease, other respiratory diseases, and congestive heart failure are considered contraindications to prolonged pneumoperitoneum. In fact, as the intra-abdominal pressure increases, the splanchnic flow, cardiac output, and renal cortical blood flow all decrease.97 An alternative for these patients may include the use of nitrous oxide or helium. However, one must consider that the former is combustible and the latter not soluble.
Laparoscope
There is usually no role for a 0-degree laparoscope for such complex procedures as colorectal surgery. The surgeon will often need to look around adhesions rather than straight forward. The minimal degree of angulation required is 30 degrees, but sometimes 45 degrees may prove to be useful. An alternative is the deflectable tip laparoscope (Figure 19-12). The camera holder should be familiar with the device. The authors’ preference is to use a video laparoscope, as opposed to an optical system with an interphase between the instrument and the camera. In fact, the latter may be associated with fogging and sterility hazards when the hose is positioned. Furthermore, the surgeon should be aware that camera chips are located on the tip of the video laparoscope, as opposed to the classical system where the camera chip is outside the patient’s abdomen.
Camera Holder
In laparoscopic surgery, the disruption of the constrained coupling between the surgeon’s hands and eyes by the interposition of a camera, moving independently of the surgeon, is a drawback that is not encountered in conventional surgery. The camera is usually held by a junior resident or a medical student, and individual who may be unfamiliar
with the surgical procedure, who may be standing in an uncomfortable position, or who may be easily distracted. As a result, the camera may rotate away from the horizon and/or drift away from the surgical field, with the potential for increased risk of causing technical errors.78 In fact, errors that lead to injury mostly stem from misperception rather than lack of knowledge or poor judgment.220 Mechanical camera holders (passive or voice-controlled) may provide a more stable image and enable surgeons to control their own view direction.102 Several reports have suggested that voicecontrolled camera holders may offer advantages when compared with a human camera holder.118,120,144,168,212
with the surgical procedure, who may be standing in an uncomfortable position, or who may be easily distracted. As a result, the camera may rotate away from the horizon and/or drift away from the surgical field, with the potential for increased risk of causing technical errors.78 In fact, errors that lead to injury mostly stem from misperception rather than lack of knowledge or poor judgment.220 Mechanical camera holders (passive or voice-controlled) may provide a more stable image and enable surgeons to control their own view direction.102 Several reports have suggested that voicecontrolled camera holders may offer advantages when compared with a human camera holder.118,120,144,168,212
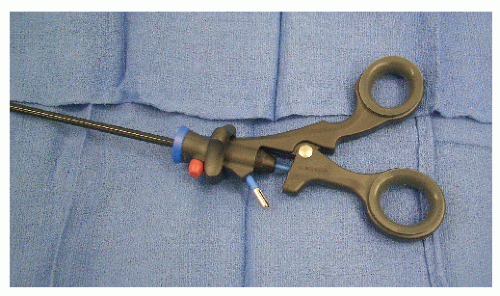
Ports
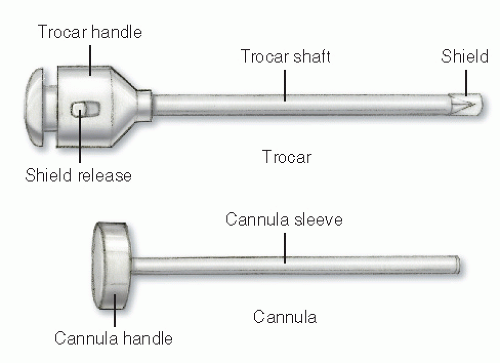
A laparoscopic port represents, in itself, a limitation of laparoscopic surgery; this is known as the fulcrum effect. A port consists of a trocar and a cannula (Figure 19-13). In general, cannula sleeves should be transparent and threaded with no metallic parts in order to allow visual control, stability within the abdominal wall, and minimal electrical risk. Bladed trocars require a retractable shield to minimize the risk of injury to intra-abdominal organs. The insertion of additional trocars does not require an open access but should be performed under direct visualization. The geometry of multiple port placements on the anterior abdominal wall should be triangular whenever possible in order to avoid injuries to the inferior epigastric vessels. Following insertion of the first port, space in the abdominal cavity may be very limited if dense adhesions are present. In such a case, bladeless trocars are not recommended because they require significant force during insertion. This may result in inward tenting of the abdominal wall. Bladed trocars or ultrasonic-guided ports (Figure 19-14) are safer for reoperative surgery because excessive force is not required during insertion.
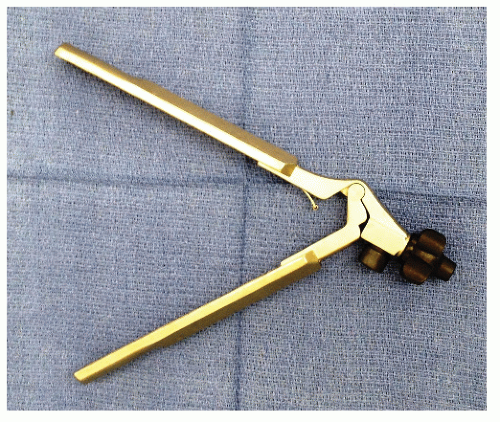
Instruments
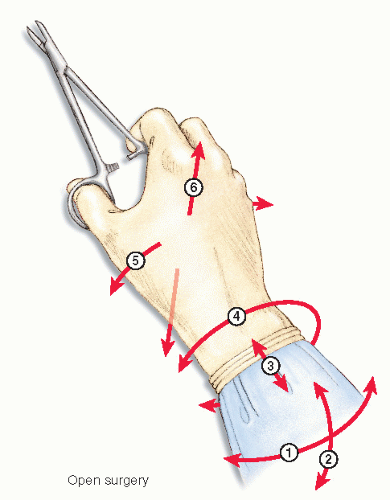
Laparoscopic instruments offer only four degrees of freedom of movement (Figure 19-15), as opposed to that of the human hand. This is obviously because of the loss of the ability to move one’s wrist (Figure 19-16). Surgeons have been traditionally using instruments with a finger loop handle, with in-line configuration to perform conventional surgery. In the early 1990s, disposable laparoscopic instruments became available, mostly with a pistol grip-shaped handle. Simulation evaluations as well as surveys report concern whether pistol grip handles meet standard ergonomic requirements.137,216 However, studies measuring surgeon forearm workload by electromyogram have been inconclusive.31,69,211 Eventually, reusable laparoscopic instruments became available, featuring various types of in-line handles.
Laparoscopic colorectal surgery may be reasonably safely accomplished with the aid of the following instruments: fenestrated bowel grasper with in-line handle (Figure 19-17) and without finger loops (Figure 19-18), right-angle clamp (Figure 19-19), curved scissors (Figure 19-20) with inline ring handle (Figure 19-21), curved-tip needle driver (Figure 19-22) with in-line ring handle (Figure 19-23), and
Satinsky vascular clamp (Figure 19-24) with in-line ring handle (Figure 19-25). Laparoscopic instruments are currently available in short (more than 30 cm) and long (more than 40 cm) shaft versions.
Satinsky vascular clamp (Figure 19-24) with in-line ring handle (Figure 19-25). Laparoscopic instruments are currently available in short (more than 30 cm) and long (more than 40 cm) shaft versions.
Dissection
Sharp dissection with cold instruments is undoubtedly the standard. Blunt dissection should be considered in laparoscopic surgery only after an areolar plane has been accessed by gentle traction applied with a laparoscopic “peanut” dissector. In the case of fused planes in between organs, surgeons should not be tempted to apply force with laparoscopic instruments and should convert to HALS or conventional open operation. The assumption is that reduced heat production may minimize thermal tissue damage.30 The use of monopolar coagulation devices should be discouraged for dissection purposes because of the high risk of thermal tissue damage. Multifunctionality and reduced heat production are among the appealing features that made ultrasonicactivated devices attractive for use in laparoscopic surgery. These allow coagulation and cutting, thereby minimizing the need for frequent instrument changes and may contribute to reducing operating time. It has been confirmed in animal models that heat production by ultrasonic-activated devices is decreased when compared with the heat generated by monopolar coagulation. The assumption is that reduced heat production may minimize thermal tissue damage.30 There are currently three ultrasonic-activated devices: AutoSonix (Tyco, Pembroke, Bermuda), SonoSurg (Olympus, Tokyo, Japan), and UltraCision (Johnson & Johnson, Cincinnati, OH)—see Table 19-1. The use of ultrasonic-activated devices near hollow organs must be discouraged because of the risk of causing injury that may not be apparent to the surgeon, such as to the small bowel and ureter. Adhesions between the anterior abdominal wall and intra-abdominal organs should be divided with cold scissors. In cases of extremely dense adhesions between small bowel loops and the anterior abdominal wall, where it is not possible to create a safe plane of dissection, it may become necessary to excise en bloc a small portion of the parietal peritoneum in order to release the bowel loop. This, of course, may be necessary even when an open operation is performed.
Hemostasis
In conventional surgery, vessels are secured with either ties or suture ligation prior to being severed. Suturing and knotting techniques in laparoscopic surgery, however, have not been embraced by the majority of surgeons. The adoption of laparoscopic clips has gained considerable popularity, but clips can limit one’s application of staplers. The inherent tedium of mastering intracorporeal knot-tying techniques probably accounts for greater interest in energy-based alternatives.
In the early 1990s, laparoscopic vessel-sealing devices became available as a safe option to divide arteries up to 7 mm in diameter (Figure 19-26). Burst pressure testing in animal models has demonstrated that 3 to 7 mm arteries managed with ties, clips, or vessel-sealing devices can withstand a maximum pressure of 900 mm Hg.110 Interestingly, the same experiment reported that 3 to 7 mm arteries managed with ultrasonic-activated devices could burst at pressures less than 100 mm Hg. Nevertheless, there are no data regarding safety of laparoscopic vessel-sealing devices when applied to large, calcified arteries.
Hemostasis can be a challenge in laparoscopic surgery. Excessive bleeding may be due to preexisting comorbidities, intraoperative findings, and such is not uncommon during reoperations. Adhesions can alter the anatomy that may complicate dissection. Medications such as cortisone, thrombocyte aggregation inhibitors, and anticoagulants can decrease tissue consistency and increase the risk of oozing that can impede the view. Bleeding due to intraoperative findings tends to occur with inadequate exposure or inability to appropriately manipulate the tissue or to equipment failure. Prevention of bleeding begins with correctly understanding the anatomy and choice of the appropriate instruments. The following materials for management of hemostasis should be available in the operating room, especially for reoperative cases: clips, collagen fleece, coagulation (bipolar and monopolar), endoloops, fibrin adhesive, sutures, and a vessel-sealing device.
TABLE 19-1 Comparison of Characteristics and Settings of Three Ultrasonic-Activated Devices | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
It should be axiomatic that every member of the operating room staff, before assisting, should attend an in-service session or sessions for all devices. The operating room team including the scrub and circulating nurses should discuss preoperatively the algorithms established to handle acute bleeding events and especially to familiarize themselves with the nomenclature of the devices. For example, in the case of a spurting vessel, the camera holder should immediately retract the telescope into the port to avoid obstruction of view. The surgeon should apply compression with his or her nondominant hand to temporarily control the bleeding. For venous bleeding, compression can be achieved with a small sponge introduced into the abdomen via a 10-mm trocar. Bleeding from arteries with a diameter of 7 mm or less can be controlled by using endoloops, clips, suture ligation with intracorporeal knot-tying, or vessel-sealing devices. Before final hemostasis is ensured, it is necessary to ascertain that the bleeding vessel is “expendable.” If the vessel is not expendable, a laparoscopic Satinsky vascular clamp should be applied temporarily to achieve vascular control while a laparotomy is performed in preparation for vascular reconstruction. Oozing from the liver, pancreas, spleen, mesentery, or retroperitoneum can be treated effectively by compression and definitive hemostasis with collagen fleece and a fibrin adhesive. Before a hemostyptic is applied, compression should be
carried out patiently for a sufficient period. In complicated reoperative procedures, use of a drain can be considered. A drain with active suction is effective, but it should not be placed directly near the source of the previously controlled bleeding site.
carried out patiently for a sufficient period. In complicated reoperative procedures, use of a drain can be considered. A drain with active suction is effective, but it should not be placed directly near the source of the previously controlled bleeding site.
 FIGURE 19-26. Laparoscopic vessel-sealing device with an in-line handle, with finger loops, and 5-mm shaft. |
Intraoperative Endoscopy
Intraoperative endoscopy is a tool that may be helpful in reoperative surgery and should preferably be made available prior to the beginning of the operation. Intraoperative gastroscopy and enteroscopy are unlikely to be indicated during reoperative surgery for colon and rectal diseases. The benefit of an intraoperative gastroscopy should always be weighed against the likely increase in small bowel distention that inevitably follows. Options for minimizing small bowel distention include applying a laparoscopic bulldog clamp (Figure 19-27) on the proximal jejunum just distal to the Treitz ligament and/or using CO2 for insufflation during endoscopy. Intraoperative enteroscopy, if indicated, should preferably be performed as push enteroscopy, given the relative ease of use and the fact that low capital investment and maintenance are involved. On the other hand, double-balloon enteroscopy requires specialized training, significant capital investment, and is time consuming. Spiral enteroscopy is still under evaluation. One advantage of positioning the patient in the lithotomy position is to make intraoperative colonoscopy easily feasible. Intraoperative colonoscopy may become necessary for the assessment of ischemia, perforation, neoplasia, anastomoses, and other indications. Again, insufflation with CO2 is to be preferred in order to prevent prolonged distention of the large bowel. Rigid proctoscopy is also a requirement in emergency colorectal cases because it is the ideal instrument for evaluating the rectum.
Closure
Following a successful performance of a laparoscopicperformed operation, the technique for closure of the ports should not be trivialized because complications may occur. Port site hernias may include an asymptomatic fascial defect or, more threatening, an incarcerated or strangulated hernia. Surgeons should always make an attempt to close all ports. Fascial defects of 10 mm or greater in diameter should always be sutured even in the case of oblique trocar insertion. Fascia closure may preferentially be performed with an open technique using a robust needle (UR-6) in order to minimize the chances of needle fracture within the abdominal wall. Open suture closure of a 5-mm port site is often not possible unless the patient has a low BMI. In most cases, it is, therefore, preferable to close a 5-mm defect laparoscopically using a suture passer. Should the patient have a preexisting large incisional hernia requiring a mesh, the repair may have to be postponed. This is particularly true if an enterotomy or bowel resection is included in the surgery.
Room Setup
Sir Charles Bell stated, “If it be a great operation, and especially if the assistants and nurses are not habituated, be careful to appoint them their places and their duties; for nothing tends more to the right performance of an operation of magnitude, than that composure and quietness which result from arrangement.” (The Principles of Surgery, London, T. Cadell & EW Davis, 1826-1828.)
The foregoing comment was made in 1821, yet it is still extremely important to the contemporary operating room scene (Figure 19-28). There is no doubt that laparoscopic colorectal procedures are “operations of magnitude.” With so many distractions of equipment— individuals, often in unfamiliar circumstances—it is essential to consider all that is required to ensure quietness and composure.
A nostalgic view of the bittersweet world of conventional surgery (Figure 19-29) is succeeded by a humorous
depiction of the perceived chaos and associated stress that accompanies the hard-won, successful transition to laparoscopic surgery (Figure 19-30). The perplexities or entanglements experienced by many surgeons when HALS becomes paramount are shown in Figure 19-31. Figure 19-32 reveals an entertainingly common, albeit unenlightened view of the field of robotics, a new technology that requires further exploration.
depiction of the perceived chaos and associated stress that accompanies the hard-won, successful transition to laparoscopic surgery (Figure 19-30). The perplexities or entanglements experienced by many surgeons when HALS becomes paramount are shown in Figure 19-31. Figure 19-32 reveals an entertainingly common, albeit unenlightened view of the field of robotics, a new technology that requires further exploration.
▶ SURGICAL PROCEDURES
Laparoscopic-Assisted Colonoscopic Polypectomy for Difficult Right Colon Polyps
Introduction
Not all right colon polyps discovered at colonoscopy are amenable to safe colonoscopic snare polypectomy. Large polyps or those positioned in a difficult location are often too risky to remove or are not completely resectable by standard snare polypectomy. As such, standard practice has been to biopsy such lesions in order to obtain a histologic diagnosis and to exclude cancer, marking the site through using a tattoo agent, and then referring the patient for right colectomy. Laparoscopic-assisted colonoscopic polypectomy (LACP) has been reported and practiced at selected centers for the management of large polyps without histologic evidence of cancer.232 This is a procedure performed in the operating room in which the surgeon and gastroenterologist work together to remove the polyp without removing the colon. Colonoscopy is accomplished using CO2 rather than air in order to allow faster desufflation of colon.152 Although LACP has been recognized as an acceptable form of treatment for the past 5 to 10 years, it has not been compared with laparoscopic right colectomy in randomized, controlled trials. Currently, throughout the United States, right colectomy for large polyps is more commonly practiced than LACP.
 FIGURE 19-30. A hyperbolic view of the hard fought transition to laparoscopic surgery. (Courtesy of Marvin L. Corman, MD.). |
Indications
Patients found to have a right colon polyp at colonoscopy in which the polyp is deemed unresectable by the gastroenterologist can be offered LACP based on the following criteria: polyp spanning two or more colonic folds, polyp >20 mm, polyp spanning greater than 50% of the colonic circumference, positive “lift sign” following submucosal injection of saline beneath the polyp, and difficult location or position of the polyp, thus limiting one’s ability to determine that removal can be adequately and completely accomplished.
Patients with the following criteria should not be offered LACP: polyps with histologic evidence of adenocarcinoma, patients with known inflammatory bowel disease, patients with known familial adenomatous polyposis, polyps without known histology, and polyps with large ulcerations or those that demonstrate a “nonlift sign” during submucosal injection at the time of colonoscopy.
 FIGURE 19-31. A humorous view of handassisted laparoscopic surgery (HALS). (Courtesy of Marvin L. Corman, MD.) |
Preoperative Workup
The preoperative workup includes colonoscopy and marking the polyp site with a tattooing agent. A mechanical bowel preparation is achieved by using polyethylene glycol ingested orally the day before surgery.
Operating Room
The operating room includes a third area for colonoscopy in addition to the classical surgery and anesthesia areas. Patients in lithotomy position receive general anesthesia with endotracheal intubation and undergo placement of adequate intravenous access, nasogastric tube, and urinary catheter. Intravenous antibiotics are given. Sequential compression devices are applied to the legs. The patient with both arms tucked is securely strapped to the table at the chest and thighs because tilting the table will be necessary. The abdomen is prepped and sterilely draped. The surgeon, assistant, and scrub nurse stand on the patient’s left side. The laparoscopy monitor is located at the patient’s right side. Colonoscopy is performed using CO2. The gastroenterologist stands in between the patient’s legs.
Surgical Technique
Pneumoperitoneum is established with carbon dioxide insufflated to a pressure of 12 mm Hg and by placement of a reusable Hasson trocar of 10 mm in diameter in the infraumbilical skin using a cutdown technique. Two reusable, threaded 5-mm ports are inserted under direct visual control in the left upper and lower quadrants lateral to the rectus muscle sheath. The table is turned into a left lateral tilt as well as into the Trendelenburg position. Laparoscopic instruments include bowel graspers, needle holders, and a laparoscopic bulldog clamp (Figure 19-27). After identification of the tattoo, a laparoscopic bulldog clamp is placed on the terminal ileum to prevent distention of the small bowel by the CO2 insufflated via the colonoscope. The laparoscopic shaft is then disconnected from the bulldog clamp, thereby allowing the surgeon to use two ports. The remainder of the operation consists of providing laparoscopic assistance to the gastroenterologist in order to accomplish a complete polypectomy. Should a full-thickness colon burn or perforation occur, the surgeon performs laparoscopic suturing with intracorporeal knot-tying. However, in selected cases, laparoscopic cecal wall excision may be an option. A leak test using CO2 insufflation through the colonoscope and immersion of the bowel segment under saline is performed. The specimen is retrieved with an endoscopic net, extracted transanally and sent to pathology.
Laparoscopic Ileocolic Resection with Intracorporeal Mesentery Division for Crohn’s Disease
Introduction
The thickened and foreshortened mesentery of the terminal ileum in patients with Crohn’s disease has been safely divided by conventional surgery for decades. The introduction of a laparoscopic-facilitated ileocolic resection entails the exteriorization of the mobilized bowel and mesentery with extracorporeal division of the mesentery. However, extracorporeal ligation of a foreshortened mesentery exteriorized through a short laparotomy can be technically difficult to accomplish.150 Still, laparoscopic intracorporeal division of the mesentery for Crohn’s disease has been successfully reported.23
Indications
Patients presenting with refractory Crohn’s disease confined to terminal ileum and cecum with or without internal fistula, localized abscess, or chronic obstruction can be managed by laparoscopic ileocolic resection unless the patient presents with massive small bowel distention (Figure 19-33). Patients are not eligible for this approach if any one of the following findings is present: frozen abdomen due to large fixed mass, recurrent Crohn’s disease following prior resection, or perforated disease requiring emergency surgery.
Preoperative Workup
A thorough history and physical exam and a preoperative consultation with a gastroenterologist specializing in inflammatory bowel disease are essential. The preoperative workup includes esophagogastroduodenoscopy, proctoscopy, colonoscopy, and CT enterography. A mechanical bowel preparation is achieved by using 2 L of polyethylene glycol ingested orally the day before surgery.
Operating Room
Patients receive general anesthesia with endotracheal intubation and undergo placement of a nasogastric tube and urinary catheter. A right ureteral stent may be placed by a urologist if considered prudent. Adequate intravenous access must be ensured. Broad-spectrum intravenous antibiotics are given. Sequential compression devices are applied to the legs. Patients are placed in the supine position with both arms tucked. Individuals should be securely strapped to the table at the chest and thighs because tilting the table will be necessary. The abdomen is prepped and sterilely draped. The surgeon and the scrub nurse stand on the patient’s left side, and the assistant stands on the patient’s right. One assistant is needed to control the camera unless a robotic camera holder is available. The television monitor is located at the patient’s right foot (Figure 19-34).
Surgical Technique
Pneumoperitoneum is induced using carbon dioxide insufflated to a pressure of 12 mm Hg by placement of a reusable Hasson trocar 10 mm in diameter in the supraumbilical skin by using a cutdown technique. A 30-degree telescope is inserted. A reusable, threaded 5-mm port is placed in the right upper quadrant lateral to the rectus muscle sheath. A disposable, threaded 12-mm port is placed in the left lower quadrant lateral to the rectus muscle sheath and caudal to the umbilicus. A reusable, threaded 5-mm port is placed on the midline above the umbilicus (Figure 19-35). The table is turned into a moderate left tilt and into a Trendelenburg position. Reusable instruments include long laparoscopic instruments (more than 40-cm long shaft), bowel graspers, scissors, right-angle forceps, needle holders, and Satinsky vascular clamp. The disposable instruments include laparoscopic stapler, bipolar vessel-sealing device, and specimen bag. Laparoscopic ileocolic resection involves a lateral-to-medial approach encompassing at least nine sequential steps:
The ligament of Treitz is identified and the small bowel inspected to the ileocecal valve. Care is taken not to grasp the intestine directly, and atraumatic bowel graspers are applied to the mesentery.
The terminal ileum is carefully inspected to rule out internal fistulas. Ileosigmoid, ileoileal, or ileovesical fistulas are repaired prior to embarking on the ileocolic resection. The site of transaction on the terminal ileum is chosen to achieve macroscopically uninvolved resection margins. The terminal ileum is transected with a laparoscopic stapler after having scored the mesentery in a window beneath it.
A lateral-to-medial mobilization of the terminal ileum and cecum off of the retroperitoneum is performed. A combination of sharp and blunt dissection is used.
The right ureter is identified by inspection and by gentle palpation with a blunt instrument if necessary.
The mesentery of the terminal ileum is transected with a bipolar vessel-sealing device or stapler near the bowel wall in a proximal to distal direction. Each staple line is meticulously inspected for evidence of bleeding prior to proceeding to the next stapler application. In case of oozing, compression is attempted first followed by oversewing with intracorporeal knot-tying if this becomes necessary.
Uncontrolled bleeding is addressed with prompt application of a Satinsky vascular clamp followed by oversewing. Care is taken to avoid dividing the ileocolic vessels in order to preserve blood supply to the ascending colon.
The cecum is transected with the laparoscopic stapler.
The ileum and the ascending colon are aligned sideto-side by a stay suture placed at their antimesenteric sides; the suture is tied intracorporeally and held by the assistant. An enterotomy and a colotomy are made at the antimesenteric sites of the staple ends of the ileum and ascending colon, respectively. A side-to-side anastomosis is fashioned with a laparoscopic stapler using one 60-mm long cartridge. Following stapler extraction, stay sutures are placed on the remaining enterotomy; this, in turn, is closed in two layers of interrupted sutures.
The mesenteric defect is left open.
The specimen is delivered via a bag through the umbilical port site. The fascia is enlarged along the midline, whereas the skin is enlarged in a curved fashion.
Postoperative Care
The nasogastric or orogastric tube can be removed in the operating room at the end of the procedure. On postoperative day 1, the patient is allowed to drink clear fluids, encouraged to ambulate, and the urinary catheter is removed. If liquids are tolerated, intravenous fluid volume is decreased, a low-fiber diet is initiated, and mobilization of the patient is increased on postoperative day 2. The patient may be advanced to a regular diet on postoperative day 3 if passing flatus and comfortable. Analgesic medication is switched from epidural to oral. On postoperative day 4, intravenous fluids are discontinued, and the patient may be discharged if comfortable and is tolerating the diet.
Opinion
An outsider to the field of surgery would probably take it for granted that surgeons have a highly developed rationale for choosing a laparoscopic approach for the management of Crohn’s disease.19 However, this is not quite true. The most likely preservation of the abdominal wall, body image,64 and small bowel obstruction rates represent all the rationalities for undertaking a laparoscopic approach.9,26 Incisions for specimen extraction and port placement should be planned in advance, keeping in mind that temporary defunctioning stomas may become necessary when severe perianal disease is present or in selected patients with refractory Crohn’s colitis. In fact, 30% of patients with colorectal Crohn’s disease may require a permanent ileostomy. Furthermore, right colectomy should be avoided because it deprives patients of useful water-absorbing colonic mucosa. Unfortunately, laparoscopic ileocolic resection does not decrease disease recurrence rates.26
Laparoscopic Right Colectomy with Intracorporeal Anastomosis for Cancer
Introduction
Although laparoscopic-assisted right colectomy was first reported in 1991,193 descriptions of a standardized surgical technique became available in recent years. The first consisted of a five-step lateral-to-medial approach with extracorporeal vascular ligation and ileocolic anastomosis.233 The second included eight steps with a medial-to-lateral approach in which vascular ligation was performed intracorporeally, whereas the anastomosis was fashioned extracorporeally.197 Laparoscopic intracorporeal right colectomy entails 10 steps with intracorporeal anastomosis followed by specimen removal in a bag.27
Indications
Resection of malignant tumors (adenocarcinomas, carcinoid tumors, neoplastic polyps) localized to the distal ileum, ileocecal valve, appendix, cecum, ascending colon, or hepatic flexure can be managed by right hemicolectomy. This procedure entails complete mobilization of the terminal ileum and right colon to the level of the midportion of the transverse colon. This technique is contraindicated for T4 right colon cancer invading the right kidney, duodenum, or abdominal wall. However, it can be used in the cases of T4 tumors invading the greater omentum.
Preoperative Evaluation
Undertaking a thorough history and performing a complete physical exam are essential. Chest x-ray and electrocardiogram (EKG) should be performed in patients older than the age of 50. Preoperative workup includes a complete blood count, blood chemistry determination, and a coagulation profile. Colonoscopy, CT scan of the abdomen and pelvis with intravenous and oral contrast, or positron emission tomography (PET) scan can establish the exact location of the malignancy and the tumor TNM grading (tumor size, lymph nodes, and systemic metastasis).
Operating Room
Patients receive general anesthesia with endotracheal intubation and undergo placement of a nasogastric tube and urinary catheter. If the individuals cannot tolerate general anesthesia, the procedure can be done with a spinal or epidural, with the addition of a transverse block. A mechanical bowel preparation is achieved by using 2 L of polyethylene glycol ingested orally during the day before surgery. Adequate intravenous access should be ensured. Broadspectrum intravenous antibiotics are given. Sequential compression devices are applied to the legs. Patients are supine in a beanbag with the right arm abducted and the left arm tucked. The patient should be securely strapped to the table at the chest because tilting the table is necessary. The abdomen is prepped and draped sterile. The surgeon , the camera holder, and the scrub nurse stand on the patient’s left side
(Figure 19-36). One assistant is needed to control the camera unless a robotic camera holder is available.
(Figure 19-36). One assistant is needed to control the camera unless a robotic camera holder is available.
Surgical Technique
Pneumoperitoneum is induced using carbon dioxide insufflated to a pressure of 12 mm Hg by placement of a reusable Hasson trocar of 10 mm in diameter in the infraumbilical skin using a cutdown technique. A 30-degree telescope is introduced for peritoneal inspection. A reusable, threaded 5-mm port is placed 3 cm medial to the right anterior superior iliac spine. A disposable, threaded 12-mm port is placed in the left upper quadrant lateral to the rectus muscle sheath and above the umbilicus. A reusable, threaded 5-mm port is placed 3 cm proximal to the pubic tubercle just left to the midline. The table is turned into a moderate left tilt as well as into a Trendelenburg position. Reusable devices include long instruments (43 cm shaft): bowel graspers, scissors, rightangle forceps, and needle holders. The disposables include an ultrasonic-activated device and staplers. Laparoscopic intracorporeal right colectomy involves a medial-to-lateral approach, encompassing the following 10 sequential steps:
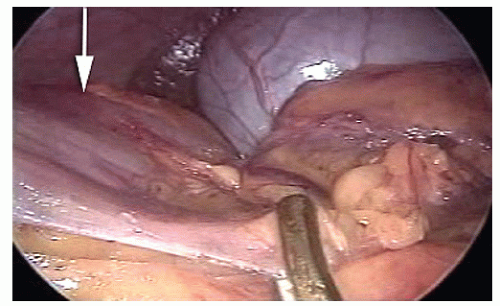
The ileocolic vessels are identified by gentle traction applied by the assistant to the cecum; the superior mesenteric vein (SMV) is located; a window beneath the ileocolic vessels is opened, incising the peritoneum close to the SMV and gently lifting the ileocolic vessels with a closed grasper; the ileocolic artery crossing to the SMV is assessed as anterior or posterior, and the ileocolic vessels were divided after the duodenum had been identified (Figure 19-37).
The right ureter is identified by inspection and, if necessary, by gentle palpation with a blunt instrument (Figure 19-38) while the assistant gently elevates the stump of the ileocolic vessels (specimen side) off the retroperitoneum (Figure 19-39).
The dissection starts at the origin of the ileocolic vessels proceeded along the SMV in a cephalad direction; if present, the right colic vessels are divided after having assessed whether the right colic artery crosses anteriorly or posteriorly to the SMV; the dissection ends at the origin of Henle’s gastrocolic trunk from the SMV; the mesentery of the proximal transverse colon is gently elevated off the duodenum (Figure 19-40).
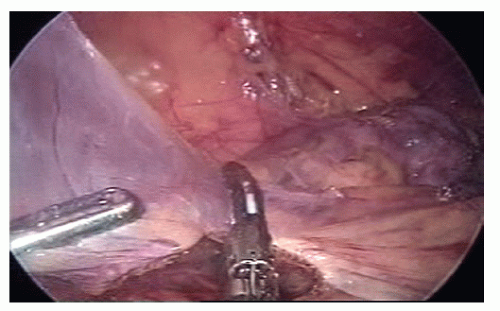
The table is leveled from the Trendelenburg position but kept in a moderate left tilt; the omentum is divided and the lesser sac entered; the division of the omentum is performed in a medial-to-lateral direction, caudal to the right gastroepiploic vessels by using an ultrasonicactivated device (Figure 19-41).
The middle colic vessels are identified by gentle elevation of the transverse colon off of the duodenum and retroperitoneum; the right branch of the middle colic vessel is divided while the proximal transverse colon is gently elevated by the assistant (Figure 19-42).
The proximal transverse colon is transected with a laparoscopic stapler (Figure 19-43).
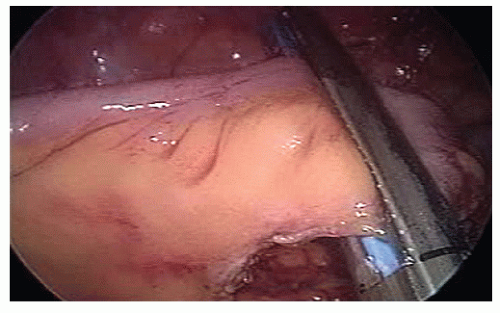
The hepatic flexure is mobilized in a medial-to-lateral direction using an ultrasonic-activated device; if present, the superior right colic vein is divided; the mesentery of the ascending colon is mobilized off the retroperitoneum, and the lateral peritoneal reflection of the ascending colon is divided along the white line of Toldt in a caudal to cephalad direction using an ultrasonicactivated device (Figure 9-44).
The terminal ileum is transected with a laparoscopic stapler and held by the assistant to prevent rotation of its mesentery (Figure 19-45).
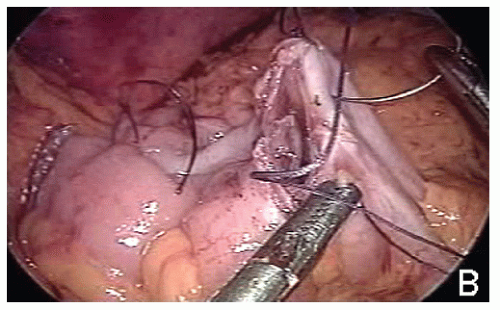
The table is leveled from the left tilt; the antimesenteric side of the stapled ends of the transverse colon and terminal ileum are approximated by a stay suture tied intracorporeally and then held by the assistant; an antimesenteric enterotomy and an antimesenteric colotomy are made 10 cm distal to the stapled ends of the transverse colon and terminal ileum, respectively; a side-toside anastomosis is fashioned with a laparoscopic stapler (Figure 19-46); the enterotomy after stapler extraction is closed by two layers of silk sutures tied intracorporeally (Figure 19-47); the mesenteric defect is left open.
The specimen is delivered in a bag through an enlarged umbilical or suprapubic port site. Fascial defects are closed with 0-polyglycolic acid sutures. Skin incisions are closed with running subcuticular 4-0 polyglycolic acid sutures.
Postoperative Care
The nasogastric or orogastric tube can be removed in the operating room at the end of the procedure. On postoperative day 1, the patient is allowed clear fluids, encouraged to ambulate, and the urinary catheter is removed. If liquids are tolerated, a low-fiber diet is commenced on postoperative day 2, intravenous fluid replacement is discontinued, analgesic medication is prescribed orally, and mobilization of the patient increased. On postoperative day 3, the patient may be discharged if comfortable, tolerating the diet, and passing gas. If patients do not tolerate a solid diet, they are not pushed to start eating, but intravenous fluid and analgesic administration are withdrawn because both of them prolong ileus.
Opinion
Potential advantages of intracorporeal ileocolic anastomosis include (1) anastomosing at some distance from the abdominal wall—this may reduce surgical site infection rates; (2) no manipulation of the abdominal cavity by the surgeon’s hands can reduce the risk of creating adhesions and perforce the rate of adhesive small bowel obstruction; (3) a 50% reduction in the abdominal wall incision length for specimen extraction could lead to clinically relevant benefits; (4) laparoscopic visualization during the creation of the anastomosis should minimize the risk of unrecognized twisting of the terminal ileal mesentery; and (5) no requirement for mobilization of the transverse colon. Intracorporeal ileocolic anastomosis following laparoscopic right colectomy may be fashioned either partially stapled, hand-sewn, or totally stapled.
Potential disadvantages of totally stapled intracorporeal ileocolic anastomosis (Figure 19-48) are as follows: (1) leads to an everted anastomosis with the potential for increased risk of complications as opposed to its hand-sewn inverted counterpart; (2) requires usage of 60-mm long cartridge for side-to-side ileocolic stapling; it inevitably excises tissue and thereby potentially reduces the size of the anastomosis; (3) most likely requires an additional port if performed on the transverse colon; (4) does not obviate the need for intracorporeal suturing because stay stitches are required to ensure safe stapler application on the enterocolotomy; (5) may require multiple firings, depending on the size of the enterocolotomy or whether the previous staple line is being excised; and (6) leads to higher costs due to additional cartridges. The author’s preferred option is to hand-sew the enterotomy (Figure 19-49).
Laparoscopic-Extended Right Hemicolectomy for Transverse Colon Cancer
Introduction
Tumors in the transverse colon pose several challenges for the surgeon. The tumors can receive blood supply from the right colic, middle colic, as well as the left colic artery, and, hence, the lymph-bearing area can be wide and variable. A segmental resection of the transverse colon can result in tension on the anastomosis secondary to the fixity of the ascending and descending colon to the retroperitoneal structures. In addition, there is an increased risk of anastomotic leak when performing colon-to-colon anastomosis as compared with ileocolic anastomosis. To avoid this and to ensure that all the lymph-bearing areas of the tumor have been removed, it is common practice to perform either an extended right or left hemicolectomy rather than a segmental resection of the transverse colon. An extended right hemicolectomy requires division of the middle colic artery, which can be challenging laparoscopically and requires advanced laparoscopic skills.
Due to these complexities, tumors of the transverse colon have been omitted from the large, randomized controlled trials that compare laparoscopic colon resection for cancer to open resection, such as COST, COLOR, and CLASICC. The results of these trials are, therefore, not directly applicable to transverse colon tumors; doubt remains regarding the optimal approach for the management of lesions in this location. Because cancer of the transverse colon is rare (10% of all colon cancer cases), unfortunately it is unlikely that separate trials will be conducted to settle this issue. However, in the literature, there are a few single-institution, nonrandomized cohort studies and quantitative comparisons that strongly suggest that laparoscopic surgery for transverse colon cancer is as safe and feasible as other laparoscopic surgeries for colon cancer.114,122,192
Indications
A tumor that is located at or just distal to the hepatic flexure can be removed by extending the classic right hemicolectomy so that it includes ligation of the right branch of middle colic artery, as long as that permits a safe, 5-cm tumor-free margin. Similarly, a tumor at or just proximal to the splenic flexure can be removed by extending the classic left hemicolectomy so that it includes ligation of the left branch of the middle colic artery. An extended right hemicolectomy with ligation of the trunk of the middle colic artery and an anastomosis between the ileum and descending colon should be used for colon cancer arising between the two flexures. This technique is contraindicated for T4 colon cancer invading adjacent organs, except for cases where only the omentum is involved.
Treatment options must be discussed with the patient. One should understand the material facts and possible risks and complications of the planned surgery before the individual signs an informed consent.
Preoperative Workup
A thorough history and physical exam are essential. Chest x-ray and EKG should be performed in patients older than the age of 50. Preoperative workup includes a complete blood count, chemistries, coagulation profile, and a carcinoembryonic antigen (CEA) level. Preoperative localization of colon cancer is always important, but for transverse colon cancers, it is imperative. Colonoscopy alone is not adequate for this determination. CT scan of chest, abdomen, and pelvis with intravenous and oral contrast and PET scan can establish the exact location of the tumor and the TNM stage, respectively. To further confirm the exact localization of the tumor, India ink injection can be performed at the time of colonoscopy. The bowel should be prepared orally with laxatives and antibiotics in accordance with each institution’s protocol, unless there is a narrow stricture. In such cases, no oral bowel preparation should be used.
Operating Room
The patient should be identified with an appropriate time out prior to the induction of general anesthesia with endotracheal intubation. Intravenous antibiotics should be administered perioperatively. A Foley catheter and nasogastric or orogastric tube should be inserted. The patient is supine on the operating table with the lower limbs either in stirrups or spread apart. Sequential compression devices are applied to the legs. Both arms should be tucked. The patient should be securely strapped to the table at the chest because tilting the table will be necessary. All equipment including monitors should be placed on the patient’s right side in clear view of the surgeon, who stands on the patient’s left side. One assistant is needed to control the camera unless a robotic camera holder is available. The abdomen and perineum should be prepped and draped in a sterile manner.
Surgical Technique
The procedure for extended right hemicolectomy begins exactly like a standard right hemicolectomy. The instruments used are long bowel graspers, scissors, right-angle forceps, and a needle holder. The dissecting energy source can be either an ultrasonic-activated device or an electrosurgical vessel-sealing device. If an ultrasonic machine is employed, a vascular stapler should be used for the vascular pedicles. Choice of mode of access (open or laparoscopic) for the peritoneal cavity should be based on the surgeon’s experience and preference. A reusable 10-mm port is placed at the umbilicus. A reusable 5-mm port is placed 3 cm medial to the right anterior superior iliac spine. A disposable, threaded 12-mm port is placed in the left upper quadrant lateral to the rectus muscle sheath and cephalad to the umbilicus. A reusable 12-mm port is placed 3 cm proximal to the pubic tubercle just to the left of the midline. A 30-degree videoscope (5 or 10 mm in diameter) should be placed at the umbilicus.
Step 1: The surgeon and assistant begin by standing on the patient’s left side. The table is turned into a moderate left tilt as well as into a slight Trendelenburg position. The peritoneal surfaces and liver are inspected for tumor growth. The ileocolic vessels are identified by gentle traction applied by the assistant to the cecum, and the SMV is located. A window beneath the ileocolic vessels is opened incising the peritoneum close to the SMV and gently lifting the ileocolic vessels with a closed grasper; the ileocolic artery crossing to the SMV is assessed as anterior or posterior, and the ileocolic vessels are divided after the duodenum has been identified.
Step 2: The right ureter is identified by inspection and, if necessary, by gentle palpation with a blunt instrument while the assistant gently elevates the stump of the ileocolic vessels (specimen side) off of the retroperitoneum.
Step 3: The dissection starts at the origin of the ileocolic vessels and proceeds along the SMV in a cephalad direction; if present, the right colic vessels are divided after one has assessed whether the right colic artery crosses anteriorly or posteriorly to the SMV. At this point, the dissection ends at the origin of the Henle’s gastrocolic trunk from the SMV. The mesentery of the proximal transverse colon is gently elevated off the duodenum.
Step 4: The table is leveled from the Trendelenburg position but kept in a moderate left tilt; the omentum is divided and the lesser sac entered; the division of the omentum is performed in a medial-to-lateral direction caudal to the right gastroepiploic vessels using a dissecting device.
Step 5: The hepatic flexure is mobilized in a medial-to-lateral direction, the lateral peritoneal reflection of the ascending colon is divided along the white line of Toldt in a caudal to cephalad direction, and the mesentery of the ascending colon is mobilized off the retroperitoneum.
Step 6: The terminal ileum is transected with a laparoscopic stapler, and the pole of the cecum is mobilized.
Step 7: The surgeon and assistant now move to the patient’s right side. The surgeon uses the right-sided and suprapubic ports. The table is tilted to the right and in reversed Trendelenburg position, and the transverse colon is gently elevated off the duodenum to identify the middle colic vessels. It is important to recognize the anatomic location of the SMV as well as the gastrocolic trunk of Henle.” These vessels are short, and excessive traction can lead to profuse bleeding at the inferior border of the pancreas. The middle colic vessels are divided while the transverse mesentery is held up.
Step 8: Division of the greater omentum is continued toward the patient’s left by grasping the gastrocolic ligament, thus fully opening the lesser sac and always avoiding traction on the spleen. The splenic flexure is mobilized laterally by retracting the colon gently medially, dividing the lateral attachments, and lifting the colon off of the retroperitoneum, carefully avoiding the tail of the pancreas. To fully mobilize the flexure, the retroperitoneum is incised 1 cm below the pancreas, and the colon is peeled off of Gerota’s fascia.
Step 9: The descending colon is transected with a laparoscopic stapler, coming in from the right-sided port.
Step 10: The table is leveled from the right tilt; the antimesenteric side of the stapled ends of the descending colon and terminal ileum are approximated by a stay suture tied intracorporeally and then held by the assistant. An antimesenteric enterotomy and an antimesenteric colotomy are made 10 cm distal to the stapled ends of the transverse colon and terminal ileum, respectively, and a side-to-side anastomosis is fashioned with a laparoscopic stapler, placed at the suprapubic port. The enterotomy after stapler extraction is closed by two layers of silk sutures tied intracorporeally. The mesenteric defect is left open.
Step 11: The specimen is delivered in a bag, or using a wound protector, through an enlarged umbilical or suprapubic port site. Fascial defects larger than 5 mm are closed with 0-polyglycolic acid sutures. Skin incisions are closed with running subcuticular 4-0 polyglycolic acid sutures.
Postoperative Care
If a nasogastric or orogastric tube is used intraoperatively, this should be removed at the end of the procedure. The patient is encouraged to ambulate on the day of the procedure because early mobilization is imperative for helping to prevent complications, such as atelectasis, pneumonia, or vein thrombosis. A liquid diet should be started on postoperative day 1 if there are no clinical signs of ileus. Usually, oral pain medications are tolerated on postoperative day 1. The Foley catheter should also be removed on postoperative day 1 unless there are clinical signs of hypovolemia. Most patients are ready for discharge from the hospital on postoperative days 3 to 4. Follow-up should be at 2 and 6 weeks and after that according to each institution’s protocol for colon cancer.
Laparoscopic-Assisted Left Colon Resection for Recurrent Diverticulitis
Introduction
The natural history of diverticulitis was described in the first half of the last century. Parks observed in 1969,163 in his review of more than 500 patients, that 10% to 25% of those with diverticular disease will develop symptoms at some time in their lives. Furthermore, nearly one-fourth of those successfully treated medically after a first episode of acute diverticulitis were readmitted with a second episode. Parks also found that the disease behaved more aggressively in younger patients. Subsequent studies confirm this as well as suggesting that recurrent episodes of uncomplicated diverticulitis are a risk factor for developing complicated diverticulitis. This has led to guidelines that were followed during the second half of the last century, in which elective surgery was recommended following two episodes of acute diverticulitis and after one episode in patients younger than 40 years of age. More recent studies have challenged this approach. Simply stated it appears that the era of recommending elective surgery based on the strict criteria of the number of prior episodes and the patient’s age appears to have passed. We have now begun an era of individualized treatment.
Guidelines from major surgical societies (European Association for Endoscopic Surgery [EAES],117 American Society of Colon and Rectal Surgeons [ASCRS], and the Society for Surgery of the Alimentary Tract [SSAT]) agree that for elective resection for recurrent, uncomplicated diverticulitis, one must consider the risks of death using the colorectal POSSUM score and to what extent the frequency and severity of the episode(s) and/or persisting symptoms following the acute episode impact one’s quality of life. Because the sigmoid colon is most frequently the site of disease, a complete sigmoid resection is recommended. It is important that the level of the distal resection is accomplished in the upper rectum and not the distal sigmoid colon in order to reduce the risk of recurrence.208 There is no need to extend the resection proximally, even if there are diverticula present in the proximal colon, as long as they are not inflamed.228 There is general consensus that laparoscopic surgery for diverticular disease is safe and even advantageous; this has been confirmed in a recent randomized, controlled trial.115
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree