14
Kidney and Ureter Calculi
Omar M. Aboumarzouk1,2, Paul Cook3, Olivier Traxer5, Palle J.S. Osther4, Luca Villa5, Jonathan Cloutier5, Helene Jung4, Kim H. Andreassen4, and Bhaskar K. Somani6
1 Glasgow Urological Research Unit, Department of Urology, Queen Elizabeth University Hospital, Glasgow, UK
2 University of Glasgow, School of Medicine, Dentistry & Nursing, , Glasgow, UK
3 Department of Biochemical Pathology, University hospital Southampton NHS Trust, Southampton, UK
4 Urological Research Center, Department of Urology, Fredericia Hospital, Institute of Regional Health Services Research, University of Southern Denmark, Fredericia, Denmark
5 Urology Department, Tenon Hospital, Pierre and Marie Curie University, Paris, France
6 University Hospital Southampton, NHS Trust, Southampton, UK
Abstract
Urinary stones are an ailment that can leave the patients asymptomatic or lead to significant morbidity or even mortality. Understanding the basic concepts of stone formation is vital in its prevention, which is key not only as a preventative measure but also to ensure patients do not form further stones. In this chapter, we discuss the pathophysiology of stone formation as well as the diagnostic and management modalities.
Keywords: urinary stones; urinary calculi; renal stones; renal calculi; renal colic; noncontrast computed tomography lithotripsy; ureteroscopy (URS); percutaneous nephrolithotomy (PCNL); urinary diversion; stone‐free rate (SFR); residual fragments (RF)
14.1 Epidemiology
Renal stone disease is common, with a worldwide prevalence of between 2 and 20%. Epidemiological studies in the United States show a lifetime risk for men of 12% and for women 5%. Stone recurrence is also common; it is estimated that almost 10% with a stone will form another stone within a year and nearly 50% of stone formers will have a recurrence within 10 years [1]. Recurrent stone formers also have lower estimated glomerular filtration rates (eGFR) when compared to non‐stone formers matched for age, sex, race, and body mass index (BMI) [2].
14.2 Pathophysiology
Stones in the urinary tract are usually predominantly crystalline. Crystallisation is a physicochemical process involving a change of phase in which dissolved salts condense into solids – all phase changes are driven by supersaturation. At supersaturation values less than one crystals of a substance will dissolve, whereas values greater than one crystals form and grow. Urine with supersaturation values greater than one is referred to as metastable because the excess dissolved material, being present at a concentration above its solubility, must eventually precipitate. The upper limit of metastability (also called the ‘formation product’) is lower amongst patients with stones, indicating that mechanisms protect against solid‐phase development are less effective in patients with stones [3].
There are three important processes for crystallisation: crystal nucleation, crystal growth, and crystal aggregation. All three of these processes are dependent on the degree of supersaturation.
It is not enough that crystals should precipitate in the urine because loose grains would be flushed out the next time the patient passed urine. Another factor is always present, the protein matrix, which glues particles together into a coherent mass. Matrix forms from 2.5 to 60% of the dry weight of stones, even those in which supersaturation is the most obvious underlying factor (e.g. uric acid and cystine calculi).
Urine contains molecules that retard the formation of solid phases (i.e. reaching formation product). Urinary citrate binds calcium to form a soluble complex and inhibits nucleation and growth of calcium crystals. Osteopontin (uropontin), calgranulin, Tamm Horsfall glycoprotein, and glycosaminoglycans can all bind with surface calcium atoms and prevent crystal growth [3]. Urine pH is also a modulator of stone formation because some crystals such as calcium phosphate are more soluble in acid urine, whereas uric acid and cystine are more soluble in alkaline urine (Figure 14.1).

Figure 14.1 Effects of increasing ion concentration in the urine.
14.3 Formation of Calculi in the Kidney
14.3.1 Concretions
Most stones that form in a papilla begin as multiple minute calcium oxalate stones (Figure 14.2). Later, an accumulation of these minute concretions forms a layer under the tip of the papilla – Randall’s plaque. This subsequently separates into the lumen of the calyx.

Figure 14.2 Most calculi originate in the kidney as Carr’s concretions in the collecting tubules, which accumulate to form Randall’s plaques in the papillae, which finally work loose.
14.3.2 Papillary Necrosis
A second mechanism for stone formation in the renal papilla is ischaemic necrosis seen with many of the conditions that cause interstitial nephritis. The dead papilla acts as a nucleus for secondary accumulation of calcium oxalate or struvite.
14.3.3 Medullary Sponge Kidney
In medullary sponge kidney (MSK), the renal collecting tubules become grossly dilated. Stones may form as a result of stasis, but there may also be an element of renal tubular acidosis. The condition may appear in one calyx and gradually spread through the kidney. Its cause is unknown, but it is associated with unilateral hemi‐hypertrophy and other congenital conditions.
14.3.4 Hydronephrosis and Hydrocalyx
A fourth type of stone is seen in a chronically dilated renal pelvis or calyx; these stones are usually multiple and rounded and may be so numerous that they are called ‘milk of calcium stones’.
14.3.5 Recumbency Stones
In spinal injury and major trauma, stone formation was once a common complication. There were several contributing factors: hypercalciuria from loss of calcium from the skeleton resulting from inactivity, urinary infection, and dehydration. Frequent turning, early mobilisation, and active treatment of infection can largely prevent this condition.
14.4 Common Types of Urinary Stones
Against this background, it is appropriate to consider some of the different types of stone (Table 14.1).
Table 14.1 Types of stones.
| Main composition of stone | Percentage of all stones |
| Calcium oxalate | 60–85% |
| Struvite (infection or triple phosphate stones) | 1–20% |
| Calcium phosphate | 1–10% |
| Uric acid | 5–10% |
| Cystine | 1% |
14.4.1 Calcium Stones
The most common type of stone is made of calcium oxalate occurring in approximately 60–80% of patients, with calcium phosphate stones making up 10% [4]. The majority of calcium oxalate stones form on the surfaces of renal papillae over collections of suburothelial calcium phosphate particles known as Randall’s plaques. The origin of the plaques is the basement membrane of the deep thin loops of Henle [5].
Common biochemical risk factors for calcium oxalate stones include hypercalciuria, hypercalcaemia, and hyperoxaluria. Along with urine volume, urine calcium and oxalate concentrations are the main determinants of calcium oxalate supersaturation.
Idiopathic hypercalciuria is the most common metabolic abnormality in calcium oxalate stone formers. Diagnosis of idiopathic hypercalciuria requires exclusion of conditions such as hypercalcaemia, sarcoidosis, and rare monogenic disorders such as Dent disease. High dietary sodium also increases urinary calcium excretion as does excessive protein intake [6].
Urinary oxalate is a critical factor in calcium oxalate stone formation because its concentration is much less than that of urinary calcium, and so a small decrease will have a greater impact on reduction in stone risk than a reduction in urinary calcium.
Oxalate is a metabolic end product of no known use. Its origins are either exogenous (i.e. dietary) or endogenous (i.e. metabolic). Foods high in oxalate content include, tea, coffee, dark chocolate, rhubarb, berries, and spinach [7]. Hyperoxaluria causing renal stones is seen in three main forms:
- Dietary causes of hyperoxaluria include excessive intake of foods high in oxalate content, a low calcium intake because of reduced calcium oxalate crystallisation in the gastrointestinal lumen, and a high animal protein intake.
- Enteric hyperoxaluria occurs in conditions that are associated with fat or bile acid malabsorption, such as inflammatory bowel disease, pancreatic insufficiency, or bowel syndromes, such bowel resection, jejunaoileal bypass, and Roux‐en‐Y gastric bypass. Hyperoxaluria complicating Roux‐en‐Y gastric bypass is under‐recognised [8]. In the normal state, calcium and oxalate within the lumen of the intestine combine to form insoluble calcium oxalate complexes that are excreted in faeces. In fat malabsorption and enteric hyperoxaluria, excessive intraluminal free fatty acids bind to and saponify calcium within the intestine, thereby inhibiting the formation of calcium oxalate. As a result, greater quantities of soluble‐free oxalate are absorbed by the colonic mucosa (Figure 14.3). In addition, free fatty acids and bile salts enhance the colonic mucosa’s permeability to oxalate.
- Genetic: The primary hyperoxalurias are a rare group of autosomal recessive disorders. There are three forms of primary hyperoxaluria in which the underlying defects have been identified. Each is caused by an enzyme deficiency, and each affects a different intracellular organelle. Primary hyperoxaluria can occur at almost any age from birth to the sixth decade of life. The clinical presentation varies from infantile nephrocalcinosis to occasional stone formation in adulthood. However, 20–50% of patients have advanced chronic kidney disease at the time of diagnosis. Progressive systemic involvement can occur with major sites of crystal deposition being the kidneys, blood vessel walls, and bones [9].

Figure 14.3 Hyperoxaluria from disease or loss of the terminal ileum.
Hyperoxaluria also occurs with ethylene glycol poisoning and overdose of ascorbic acid.
When calcium phosphate becomes the main constituent of a stone, it is classified as a calcium phosphate stone. However, pure calcium phosphate is rare. The most important calcium phosphates involved in urinary stone disease are apatite and brushite (i.e. calcium hydrogen phosphate dihydrate) stones. Although both minerals contain calcium and phosphate, they form as a consequence of very different mechanisms. Apatite is often found in association with struvite as a consequence of infection (see below). Pure brushite stones typically form in distal (type 1) renal tubular acidosis where there is a defect in renal acid excretion and an inability to reduce urine pH below 5.5 [10]. In the complete form, there is a systemic acidosis frequently accompanied by hypokalaemia, hypercalciuria, and hypocitraturia. The incomplete form is more common and under‐recognised and is not associated with metabolic acidosis.
14.4.2 Struvite Stones or Infections Stones
Struvite calculi are typically referred to as infection stones because of their association with urinary tract infections with urease‐producing bacteria. The most important urease producers include Proteus, Pseudomonas, Klebsiella, and Staphylococcus spp. [11]. Bacteria‐produced urease breaks down urinary urea into ammonia and carbon dioxide, which then hydrolyses to ammonium ions and bicarbonate. Binding to available cations then produces carbonate apatite and magnesium ammonium phosphate hexahydrate (struvite). Carbonate apatite crystals start to form when the urine pH is greater than 6.8, but formation of struvite crystals require an alkaline urine (pH > 7.2) and ammonia in the urine. However, formation of a biofilm is also important contributing to the stone matrix within which bacteria adhere.
Infection stones are characterised by their large size and exceptionally rapid growth. As little as four to six weeks may be sufficient for an infection stone to form and to develop into a staghorn stone that involves the entire renal pelvis or calyces.
14.4.3 Uric Acid Stones
Uric acid stones account for approximately 5–10% of kidney stones. Uric acid is filtered in the glomeruli, reabsorbed in the proximal tubule, and secreted again in the distal tubule. Uric acid is the end result of purine metabolism and is relatively insoluble. Furthermore, urine pH is a key regulator of uric acid solubility in urine with an unduly acid urine (pH < 5.5) an invariable feature in uric acid stone formers.
Contrary to expectation, unless massive, hyperuricosuria does not appear to be a significant risk factor for uric acid stones [12]. The explanation of the acidification defect in idiopathic uric acid stone formation is uncertain. Epidemiological and clinical data have demonstrated an association between uric acid stone formation and diabetes, glucose intolerance, metabolic syndrome, and obesity. The evidence from clinical studies suggests this association may in part be related to insulin resistance [13].
14.4.4 Cystine Stones
Cystinuria is the purest example of the simple supersaturation stone. They are the hardest type of stones due to their disulphide bonds. Cystinuria is responsible for 1% of adult and 6–8% of paediatric stone disease. It is an inherited autosomal recessive disorder of a heterodimic amino acid transporter resulting in decreased absorption of cystine from the intestine and the proximal tubule of the kidney. Mutations in SLC3A1 (chromosome 2) and SLC7A9 (chromosome 19) are known to cause cystinuria resulting in impairment of reabsorption of the dibasic amino acids cystine, ornithine, lysine, and arginine [14]. Although all of these amino acids reach high concentrations in the urine, only cystine is insoluble enough to form stones. Type A cystinuria refers to patients with mutations in SLC3A1 and type B where there are mutations in SLC7A9.
In heterozygote patients’ urine excretion of cystine depends on type; in type A, cystine excretion is normal, whereas in type B, cystine excretion is increased and patients may form stones. The incidence of stone formation increases when urinary cystine concentration exceeds 170 mg l. Cystine is also poorly soluble at physiological urine pH.
14.4.5 Drug‐Induced Stones
These are rare and occur in less than 1% of all stones analysed. Stones are either made of the drug, or drug metabolite, or form as a consequence of the metabolic effect of the drug. Contemporary drugs that can be found in stones include protease inhibitors and sulphonamides such as sulfadiazine. Carbonic anhydrase inhibitors, topiramate, and antacid preparations containing calcium carbonate or calcium phosphate are examples of drugs that cause stones through their metabolic effects.
14.5 Clinical Features
Stones present in a number of ways:
- Asymptomatically
- Visible or nonvisible haematuria
- Renal or loin pain
- Ureteric colic pain
- Recurrent infections
- Obstruction with or without an acute kidney injury or sepsis
14.5.1 Kidney
Pain in the kidney is experienced in the distribution of the dermatomes T9–T11, in the loin radiating down to the testicle and groin. When there is acute dilatation, the pain is colicky; in other circumstances, the stone may be entirely silent or cause a vague backache.
Sometimes it is difficult to believe that a small caliceal calculus, without any dilatation, could possibly give rise to pain. Nevertheless, experience shows that removing such a stone may relieve the pain permanently.
Some of the least obtrusive of all stones are the giant staghorn calculi, which are nearly always made of struvite. These stones may be silent, but they are not safe, and the risks of pyonephrosis and sepsis far outweigh the risks even of open surgery, let alone those of combinations of percutaneous nephrolithotomy (PCNL) and shockwave lithotripsy (SWL).
14.5.2 Ureter
Acute ureteric colic strikes without warning. The pain comes in waves. Dysfunction of the overlying intestine – probably caused by extravasation of urine into the retroperitoneal tissues – leads to vomiting and dilatation of the bowel, which may mimic intestinal obstruction and has led to many a mistaken laparotomy.
There is often a trace of blood in the urine passed at the time of the attack.
If there is infection in the urine, there may be septicaemia with all its sequelae.
14.5.3 Bladder
Stones in the bladder give pain referred typically to the tip of the penis. It is relieved by lying down and made worse by standing up and moving about. There is frequency and pain on voiding, and the last drops of urine are often bloodstained. Most patients with a bladder calculus also have long‐standing prostatic outflow obstruction, whose symptoms predominate, and the stone being found only by chance in the course of investigation.
14.6 Complications of Stones
14.6.1 Renal Stones
The most important complication of a stone is obstruction, giving rise to acute or chronic hydronephrosis. When this is further complicated by infection, there may be septicaemia, cortical abscesses, perirenal abscess, erosion into the bowel, and xanthogranuloma. Very rarely metaplasia in the urothelium adjacent to a chronic stone leads to squamous cell carcinoma or adenocarcinoma.
14.6.2 Stones in the Ureter
With a complete obstruction, there is a risk of septicaemia if the urine is infected because of the backflow of urine into renal veins and lymphatics. There is also a remote but well‐documented risk of reflex renal shutdown from spasm of the contralateral calices and ureter. In the course of time, oedema of the wall of the ureter can develop around the stone, however small, which may mimic a urothelial cancer of the ureter.
Stones which get stuck in the ureter for many months may form little pockets past which urine flows without impediment. Sometimes these become infected and erode into adjacent viscera such as the appendix or fallopian tube.
14.6.3 Stones in the Bladder
Most stones which are small enough to pass out of the ureter into the bladder will be voided via the urethra. Stones that start off in the ureter linger in the bladder, act as a nucleus for the deposition of successive shells of calcium oxalate or struvite and grow so large that it is impossible for them to pass through the urethra.
Bladder stones can be seen in men when there is existing outflow obstruction. In women, there is usually a foreign body at the centre of a stone (e.g. a fragment of a Foley catheter or a nonabsorbable suture).
Just as the trauma of a stone gives rise to metaplasia of the urothelium in the kidney, so also in the bladder, long‐standing calculi may lead to squamous metaplasia and cancer.
14.6.4 Stones in the Prostate
These arise in dilated prostatic ducts, usually on a nucleus of corpora amylacea and are often secondarily infected.
14.6.5 Stones in the Urethra
Urethral stones are still common in the tropics. The stone travels through the ureter, grows a little in the bladder, and then gets stuck at the external urinary meatus. Stones are also seen in urethral diverticula, as in any other undrained pocket of urine.
14.7 Investigations
Investigation of a patient with symptoms suggesting a stone in any part of the urinary tract has three objectives:
- to make sure that the shadow is indeed a stone;
- to find out what trouble the stone is causing, so that it can be corrected; and
- to stop it from happening again.
14.7.1 Is the Shadow Really a Stone?
In the kidney, it is easy to mistake a calcified renal artery aneurysm or calcification in the lymph nodes for a stone. Calcification in the wall of the bladder or ureter in schistosomiasis can resemble a stone as can that caused by another extraordinary fluke (Paragonimus westermani).
Phleboliths in the pelvis exactly mimic a calculus, although a computed tomography (CT) view with contrast medium or oblique X‐rays with a catheter in the ureter will show the shadow well away from the ureter. A calcified fibroid, teeth in an ovarian dermoid, or a large stone in the ureter or prostate may all resemble stones in the bladder.
14.7.2 What Trouble Is the Stone Causing?
Urography is still the key to the diagnosis of any stone in the urinary tract because most stones are radio‐opaque, and most obstruction is revealed by dilatation in the urogram.
The catch is the radiolucent stone. For practical purposes, these are uric acid stones, although it is better to be aware of xanthine stones in patients who have received protracted treatment for gout, and the excessively rare, silicate stones in patients given long courses of magnesium trisilicate for indigestion. The difficulty is quickly solved by ultrasound, supplemented where necessary by CT.
If the stone is more than 5 mm in diameter, its chance of leaving the renal pelvis or completing its journey down the ureter is so small that it ought to be removed. A small stone in an outlying calix, in the absence of infection, can be safely monitored.
14.7.3 Renal Function
An error may arise if a contrasted scan is performed soon after the onset of ureteric colic when it may show no ‘function’ in the kidney. The cause of the silent urogram in acute colic is not certain; there may be spasm of the calices and ureter or obstruction to the flow of urine along the nephron, but the phenomenon is temporary and completely reversible.
Imaging plays a key role in diagnosis, treatment planning, and follow‐up of patients with urolithiasis.
Noncontrast computed tomography (NCCT) is the best investigation for diagnosing stones. It has largely replaced plain abdominal radiography of the kidney, ureter, and bladder (KUB) and intravenous pyelography (IVP) (Table 14.2). Unlike IVP, NCCT identifies stones of any composition, with the exception of stones formed by protease inhibitors, such as indinavir [15]. KUB X‐rays show stones that are largely calcium containing (i.e. radiodense or radio‐opaque). Stones that are radiolight or radiolucent stones do not show up on X‐rays, such as uric acid stones. Some stones are in between, or relatively radiolucent, and can just be identified, such as infection stones (without calcium deposits) and cystine stones.
Table 14.2 Sensitivity, specificity, and radiation exposure of different imaging modalities for diagnosis of urolithiasis.
| Imaging modality | Sensitivity | Specificity | Radiation exposurea |
| NCCT | 98% | 98% | 4.5–5 mSv |
| Low‐dose NCCT BMI < 30 kg m −2 BMI > 30 kg m −2 | 95% 50% | 97% 89% | <3 mSv |
| Ultrasonograhyb | 25% | 90% | — |
| KUB | 58% | 62% | 0.5–1 mSv |
| IVPc | 85% | 90% | 1.3–3.5 mSv |
IVP, intravenous pyelography; KUB, plain radiography of kidney, ureter and bladder; NCCT, Noncontrast computed tomography.
a Fatal cancer risk is about 1 in 2000 for a 10 mSv radiation exposure.
b Sensitivity is stone size‐dependable.
c Fatal anaphylaxis form low osmolality contrast media is about 1 in 100 000.
NCCT correctly measures stone transverse diameter but tends to overestimate cranio‐caudal length [16]. Nonetheless, it is sometimes essential to measure the whole volume. Volume = Surface area × 0.6; Surface area = length × width × 0.785 (which is: π × 0.25)
Although NCCT is not a dynamic study, it does present indirect signs of obstruction, which in the majority of cases are sufficient for safe clinical management (Figure 14.4). A reliable qualitative assessment of presence of obstruction may be achieved by adding a contrast‐enhanced phase (excretory CT) or by an IVP if needed (Figure 14.5). Beyond identifying the stone, NCCT provides significant alternate diagnoses in 10–25% of patients presenting with acute flank pain, such as acute appendicitis, ovarian cysts, or aortal aneurisms.

Figure 14.4 (a) Noncontrast computed tomography (NCCT) showing dilatation of the renal pelvis in the left kidney (blue arrow) and kidney enlargement (green arrow) suggestive of obstruction resulting in intrarenal backflow. The space around the kidney (yellow arrow) is completely dark (clean) indicating that the obstruction is not causing pyelolymphatic backflow, which is an indirect sign that the obstruction probably is not severe. (b): NCCT showing dilatation of the renal pelvis and calyces of the left kidney (blue arrow) and perirenal stranding (space around the kidney dusty) suggestive of an obstruction causing pyelolymphatic backflow. The right kidney contains a complex renal cyst with a calcified septum (red arrow).


Figure 14.5 (a) Left: Intravenous pyelography (IVP) showing an enlarged ‘white kidney’ (blue arrow), dilatation of the renal pelvis (yellow arrow), and a dilated ureter down to the level of spina ischiadica (red arrow). The stone is not clearly seen. (b) Excretory computed tomography (CT) of the same patient showing an enlarged contrast‐dense right kidney (blue line) and perirenal fluid collection indicative of fornix rupture (green arrow). In the minor pelvis the small stone is clearly seen. (The small stone passed spontaneously, and these imaging signs do not demand drainage unless the patient show signs of infection).
Isotope functional studies yield the most certain measure of obstruction [17]. Furthermore, they can assess functionality of the kidney to better determine management (Figure 14.6).


Figure 14.6 (a and b) Coronal and sagittal views of a stag horn calculi in an asymptomatic patient that resulted in an atrophied kidney, confirmed by (c) a DMSA scan.
In the acute management of urolithiasis ultrasound has been shown to be inferior to NCCT with regard to both sensitivity and specificity (Table 14.1). However, as an initial bedside investigation, ultrasound may be useful for diagnosis of obstruction and planning of subsequent diagnostic and therapeutic actions (e.g. drainage) [18].
Although magnetic resonance imaging (MRI) has the ability to detect the secondary effects of obstructive urolithiasis, MRI is unreliable with regard to identifying both renal and ureteral calculi, and at present, MRI has no role in stone evaluation. In case of younger patients, patients who are pregnant, and patients that have undergone multiple prior CT exams in which you want to avoid ionised radiation, ultrasound is the better alternative.
14.7.4 Treatment Planning
Apart from diagnosis of stones, NCCT is useful for treatment planning. It gives you information on CT attenuation values (Hounsfield Units [HU]), inner‐stone structure, and skin‐to‐stone distance (SSD), all of which have been shown to be independent predictors of stone fragility during shock wave lithotripsy (SWL).
Stones >900–1000 HU seem to predict a poorer outcome of SWL, as does a high SSD (>10 cm) [19]. Additionally, it has been shown that inhomogeneous (NCCT bone window) calcium oxalate monohydrate (COM) and cystine stones, which traditionally are considered SWL resistant, fragment much easier than homogeneous stones with equivalent crystalline composition [20, 21]. These predictors of stone fragility on NCCT may be used in the clinical setting for selecting patients for primary SWL or endourological treatment, and thereby increase efficacy of both treatment approaches (Figure 14.7) [22].

Figure 14.7 Noncontrast computed tomography (NCCT) (left) and kidneys, ureter, bladder (KUB) (mid) showing a partial staghorn stone in the upper pole of the right kidney (blue arrow). The size of the stone would according to guidelines be most suitable for percutaneous nephrolithotomy (PCNL). However, due to its inner inhomogeneous structure with void regions (white arrow), shock wave lithotripsy (SWL) was performed. The KUB on the right shows an excellent result after just one SWL session with only minor residual stones left (red arrow).
Although there are overlaps in HU values between different stone types, CT densitometry gives you a rough estimation of stone composition, which may be useful in the further clinical management of the patients (Table 14.3) [23].
Table 14.3 Approximate computed tomography‐attenuation values (Hounsfield Units) for different stone compositions at noncontract computed tomography.
| Crystalline composition | Hounsfield units (HU) |
| Uric acid | 400–600 |
| Struvite | 550–700 |
| Cystine | 650–800 |
| Capapatite | 850–1050 |
| Calcium oxalate dihydrate (COD) | 1100–1200 |
| Calcium oxalate monohydrate | 1200–1400 |
| Brushite | 1500–1800 |
Although controversy exits whether a contrast study is needed prior to stone treatment, it might be useful if stone removal is planned and the anatomy of the renal collecting system needs to be assessed [15, 17, 24–26]. For this purpose, both excretory CT (ECT) and IVP may be used. ECT provides the possibility of performing multiplanar reconstruction and three‐dimensional reformatting (i.e. 3D CT), which may be highly valuable in cases with complex anatomy or stone burden in which surgical difficulty is anticipated (Figure 14.8) [22].

Figure 14.8 Three‐dimensional computed tomography (3DCT) showing stone burden on the left, and the relation of the stones to the collecting system on the right.
14.7.4.1 Follow‐Up and Radiation Safety
It is unquestionable that the applied imaging modality has a significant impact on detection rate of residual stones and the estimated size of the residuals, which unequivocally affects clinical decision making (see Section 14.35.5). There is no currently agreed‐upon strategy for evaluation of residual stones after stone treatment. All imaging involving ionised radiation must be conducted according the as low as reasonably achievable (ALARA) principle.
Ionised radiation risk should be thoroughly considered, when planning follow‐up regimes for patients with kidney stone. Nearly a fifth of these patients receive potentially harmful radiation doses in acute and short‐term management of urolithiasis in the follow‐up setting [27]. This does not suggest that clinicians should avoid CT technology with its entire well‐documented benefits in stone disease. On the other hand, be well aware of the benefits and risks of all diagnostic procedures.
In the case of evaluating residual stones, the risks of ionised radiation should outweigh the risks of having a residual stone. This calls for selective evaluation, in which the high sensitive conventional NCCT evaluation should be restricted to those patients who have a high risk of residuals and in whom the residual stones mandate aggressive treatment, for instance patients with infection stones.
Recently, the concept of low‐dose NCCT has been introduced. A low‐dose NCCT may be defined as a CT‐examination applying less than 3 mSv for the entire examination [15, 28]. Low‐dose NCCT performs with equivalent sensitivities to conventional NCCT for diagnosis of ureteric and renal stones [29, 30], except for diagnosis of ureteric calculi in patients with a BMI > 30 kg m−2, in which sensitivity and specificity drops to 50 and 89%, respectively, compared to 95 and 97% in patients who are not obese [28].
Ultra‐low‐dose CT protocols with radiation doses close to KUB also have been developed, which opens up for using NCCT for diagnosis of urolithiasis, even in children (Figure 14.9) and patients who are pregnant [30, 31].

Figure 14.9 Ultra‐low‐dose noncontrast computed tomography (NCCT) in a 36‐month old child with bilateral stone disease. The computed tomography (CT) could be performed at a radiation dose of 0.82 mSv.
14.8 Investigations for Metabolic Stone Disease
There are few pains to compare with that of ureteric colic, and patients are anxious to be spared a second episode. Whether to investigate patients after a single episode depends on the presence or absence of risk factors.
Any of the follow is considered high‐risk category:
- Young age (<25 years).
- Recurrent kidney stones within a year apart.
- Bilateral or multiple stones.
- First‐degree family history of stones.
- History of gout or gastrointestinal disease.
- History of non‐calcium oxalate stones.
- Stones associated with diabetes mellitus.
- Single functioning kidney or renal impairment (eGFR <60 ml min−1) with stones.
14.8.1 The First Episode or a Patient with Low‐Risk Symptoms
Tests should include serum calcium, phosphate, parathyroid hormone (PTH), as well as serum creatinine and blood for HbA1c or a fasting plasma glucose level.
The stone must be analysed wherever possible. Current technology such as Fourier‐transform infrared spectroscopy makes it possible to make an accurate identification of the composition of the stone even from tiny fragments such as those obtained by sieving the urine after extracorporeal SWL.
14.8.2 The Recurrent Stone Former or a Patient with High‐Risk Symptoms
Intervention in the form of lifestyle advice and medical therapy can reduce the rate of stone recurrence, and metabolic investigation and medical treatment are important components in the clinical management of renal stone disease. This type of patient is often investigated in a dedicated metabolic stone clinic [32].
Investigations, in addition to those already noted for patients with low‐risk symptoms, should include urine for pH measurement and exclusion of cystinuria and an accurate 24‐hour collection of urine on which calcium, oxalate, urate, citrate, magnesium, and sodium excretion can be calculated.
If renal tubular acidosis is suspected, investigations for an acidification defect include the ammonium chloride (unpleasant for the patient because of nausea, vomiting, and gastric irritation) test or furosemide‐fludrocortisone test [33]. If the urine in either test acidifies (pH <5.3), distal renal tubular acidosis is excluded.
14.9 Medical Management of Stones
Fluid intake is an important intervention in all stone formers. Increasing the urine volume to at least 2 l a day can reduce recurrence rates by 40–50%. If idiopathic hypercalciuria is diagnosed, then a thiazide diuretic is prescribed. A low calcium diet is now no longer recommended and is associated with an increase in stone formation episodes. Thiazide diuretics are cheap and safe and have been shown to reduce stone recurrence rate. Calcium supplementation can be used in enteric hyperoxaluria.
Hypokalaemia should be avoided because this can cause hypocitraturia. Potassium citrate can be used in calcium oxalate stone formers with hypocitraturia. Potassium citrate is also the treatment of choice in patients with uric acid stones and renal tubular acidosis and as an adjunct in patients with cystinuria.
Pyridoxine reduces oxalate excretion in some patients with primary hyperoxaluria type I. In approximately 30% of patients with cystinuria treatment with D‐penicillamine or alpha‐mercaptopropionylglycine is required. Both of these agents are thiol derivatives, which cleave a single cystine molecule into two cysteine molecules to make a highly soluble disulphide compound. Allopurinol is prescribed for patients who have calcium oxalate stones and hyperuricosuria unresponsive to lifestyle intervention [18].
14.9.1 Management
Management of stones depends on various factors; however, it is largely dependent on site (i.e. kidney, ureter, or bladder), number and size of the stone(s), and whether it is asymptomatic or symptomatic (acute or chronic). In all settings, the patient must be assessed for pain, infection, or obstruction, which will also determine treatment needed for it.
14.9.1.1 Acute Management
In the acute setting, whether renal or ureteric stones, the main focus is to control the pain and manage the obstruction with and without infection (Figure 14.10).

Figure 14.10 infected obstructed system with a 2.5‐cm renal pelvic stone surrounded by thick matrix seen on computed tomography (CT).
14.9.1.1.1 Pain Control
Acute renal colic is an extremely painful condition requiring immediate and efficient pain management. Pain is often accompanied by nausea, vomiting, sweating, and changes in heart rate and blood pressure. This is caused by the anatomical localization of the autonomic nervous system, which is in close proximity of the visceral nerves, allowing interaction between nerves [34]. The pain sensation in renal colic is due to the obstruction of urinary flow leading to increasing wall tension in the urinary tract [34]. This stimulates the release of prostaglandins, which further increases the wall tension and intraluminal pressure. This can be seen with both renal and ureteric stones.
- Nonsteroidal anti‐inflammatory drugs (NSAIDs)
NSAIDs are first‐choice analgesia in patients with acute renal colic [35]. NSAIDs act directly on prostaglandin release and thereby decrease the renal pressure resulting in pain relief. NSAIDs are particularly potent when administered intravenously [36]. Patients with renal colic treated with NSAIDs have significant fewer colic episodes compared to patients not treated with NSAIDs [37].
Gastrointestinal bleeding and renal failure in patients with pre‐existing renal disease are well‐known side effects to prolonged use of NSAIDs, but myocardial infarction (MI) is also a recognised potential side effect, and cardiovascular disease is considered a relative contraindication to the use of NSAIDs. Patients with prior MI are at increased risk of death and recurrent MI, even during short‐term (<1 week) treatment with most NSAIDs [38]. The use of NSAIDs, therefore, should be limited to an absolute minimum in patients with cardiovascular disease. Healthy individuals with no cardiovascular history also have a dose‐dependent increase in cardiovascular events for COX‐2‐inhibitors and diclofenac [39].
- Opioids
NSAIDs have been shown to be more effective than opioids in treating renal colic [35]. Patients receiving NSAID are less likely to need further analgesia and achieve greater reductions in pain score. Opioids, particularly pethidine, are associated with a higher rate of vomiting and other adverse effects, compared to NSAIDs. In case of NSAID intolerance or contraindications for the use of NSAID, hydromorphine, pentazocine, or tramadol are second‐choice options. Combination therapy with ketorolac and morphine may be even more effective than NSAIDs or opiates alone [40].
- Alpha‐blockers
To reduce recurrent renal colic attacks, daily α‐blockers could be given in combination with analgesics [41]. Alpha‐blockers are generally well tolerated and reduce the frequency of renal colics in patients with ureteral stones.
14.9.1.1.2 Acute Urinary Drainage
Urinary obstruction and accompanying infection due to calculi is an emergency condition requiring immediate decompression of the collecting system. Stones can not only obstruct the ureteric drainage, but also block intrarenal calyces or a calyceal diverticulum.
Patient stabilisation with conservative measures should not be delayed: fluids; analgesics; antibiotics; basic investigations: routine blood tests, clotting, and cultures; and followed by immediate CT to confirm diagnosis. If obstruction is confirmed, decompression relief is the only way to improve the patient.
Placement of a percutaneous nephrostomy (PCN) tube with or without a retrograde insertion of a JJ‐stent are both well‐established drainage procedures, but the optimal method of decompression is controversial. Appropriate antibiotic treatment should always be initialized immediately, and the definitive stone treatment should be delayed until infection has been successfully treated.
PCN avoids general anaesthesia and allows placement of a large calibre drainage tube to secure optimal decompression; however, its potential disadvantages are leakage, dislodgement of the tube, and bleeding complications, particularly in patients who are coagulopathic [42].
Insertion of a JJ‐stent demands general anaesthesia in most cases, which may have disadvantages in certain patients with severe comorbidity. Very large ureteral calculi may hamper placement of a stent, and furthermore, the presence of a JJ‐stent has been shown to reduce quality of life (QoL) in up to 80% of patients because of pain, haematuria, incontinence, sexual dysfunction, and general discomfort [43]. Anticholinergics and α‐blockers may relieve the discomfort of JJ‐stenting [44].
From a patient’s perspective, there is no difference in clinical efficacy, availability, or preference between PCN and JJ‐stenting for drainage of the obstructed infected urinary system [45]. PCN is less costly but requires longer procedural and fluoroscopy times. PCN is also safer in patients with sepsis and with less risk of complications [42].
Complication rate of PCN is about 4%, and all major complications are related to haemorrhage [46]. Complications related to stent insertion are poorly reported; however, they are related to worsening sepsis. Nonetheless, there is little evidence to suggest the superiority of PCN over retrograde stenting as primary treatment of infected hydronephrosis.
The best modality of decompression is still a matter of debate, depending on several factors including stone size and location, operators’ experience, patient preference and comorbidity, available equipment and manpower, and related costs. However, in an acute setting with a patient who is moribund, most will proceed to PCN to reduce risk of worsening septic and anaesthetic complications due to the infection.
14.10 Surgery for Stones
Treatment modalities can vary and can depend on patient, stone, and experience factors. Figures 14.11 and 14.12 depict the general consensus for stone management [25, 26].
- Observational and conservative management
- Shock wave lithotripsy (SWL)
- Ureteroscopy (URS; rigid/flexible)
- Percutaneous nephrolithotomy (PCNL)
- Laparoscopy or open stone surgery
(Source: Adapted from EAU guidelines.)
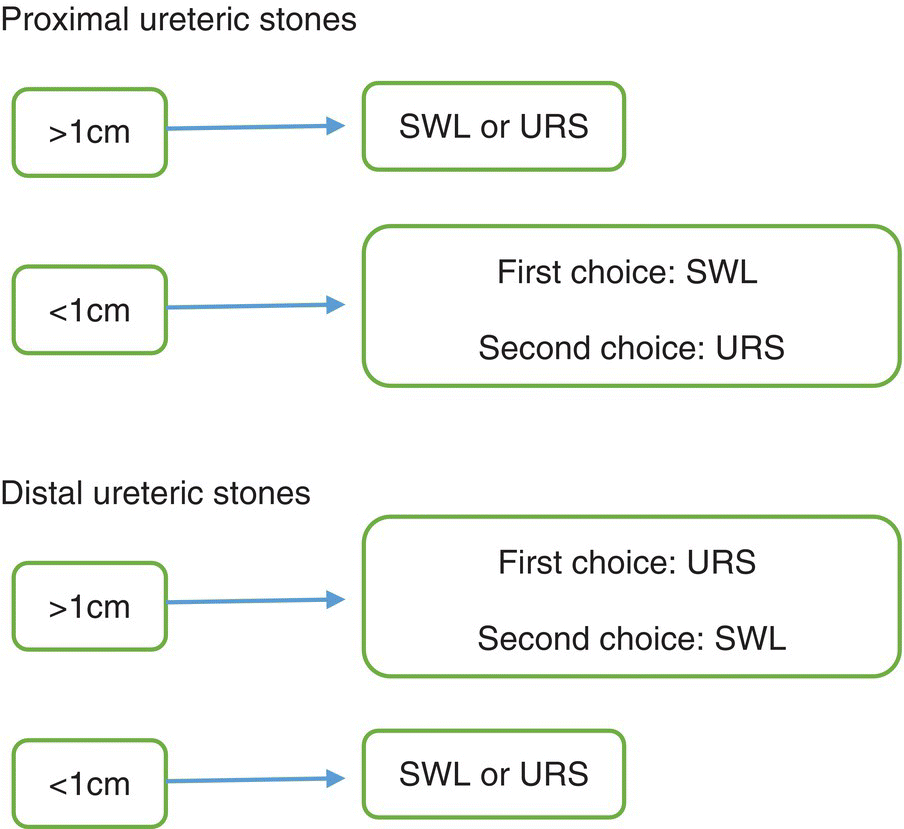
Figure 14.12 Treatment algorithm for ureteric calculi.
(Source: Adapted from EAU guidelines.)
14.10.1 Observational and Conservative Management
When treating stones, the patient needs to be treated as a whole. In other words, patients who are elderly or those with significant comorbidities with asymptomatic stones do not necessarily need treatment for their renal stones. On the other hand, obstructing ureteric stones need treatment in the acute seeing, even if there is no pain. Chronically obstructing stones that have atrophied the kidney can be left alone, if there are no symptoms. In summary, treatment of stones needs to take into consideration every aspect of the patient, not just the pathology of the stone.
Taking that into consideration, there is an overall risk of stones causing complications when patients were initially asymptomatic. However, only about 10–40% of patients will require surgical intervention within three years of renal stones, if deemed suitable for conservative management (Table 14.4) [47–49]. Stones in the lower pole are more likely to increase in size, cause symptoms, and lead to an intervention [49].
Table 14.4 Risk of stone to require intervention, or cause pain, or increase in size.
| Stones <5 mm | 5–10 mm | 11–15 mm | >15 mm | |
| Causing pain (%) | 40 | 40 | 40 | 60 |
| Increase in size (%) | 50 | 55 | 60 | 70 |
| Requiring intervention (%) | 20 | 25 | 40 | 30 |
Conversely, staghorn stones, especially infective struvite stones, need intervention unless the risk of management is high. Staghorn stones will cause damage and atrophy to the kidney and have a high risk of sepsis‐related illnesses, renal deterioration, and death [50, 51]. Renal deterioration is seen in nearly a third of patients treated conservatively [51]. With associated death ranging between 27 and 67%, patients will die as a consequence of a staghorn calculi if left untreated [50, 51]. As opposed to 3–7% mortality associated with intervention, reducing to 0% if complete clearance was achieved [50, 51].
In ureteric calculi, the majority will pass spontaneously if left untreated within three to six weeks; those <5 mm have 70–90% chance of passing spontaneously and those >5 mm have a 50–60% chance of passing with no intervention [52, 53]. If a stone did not pass after six to eight weeks, then it will be highly unlikely it will pass, and intervention is most likely required to remove it.
14.10.2 Medical Expulsive Therapy (MET)
The rate of spontaneous passage of symptomatic ureteral calculi depends on the time, location, and size of the stone. Drugs capable of relaxing the ureteral smooth muscle cells might increase the frequency of, and reduce the time to, spontaneous stone passage. MET is typically acting through α‐1 receptor blockade or inhibition of the calcium channel pumps. Treatment with α‐blockers or calcium‐channel‐blockers increases the overall chance of spontaneous stone passage by 65% [53–55]. However, MET should be used only when there is no obvious advantage from immediate active stone relief (i.e. persistent renal colic, impaired kidney function or urinary tract infection due to urinary obstruction), and MET should never delay appropriate treatment and acute urinary drainage.
14.10.2.1 Choice of Drug
Alpha‐blockers and calcium‐antagonists equally augment stone expulsion rates, reduce time to stone expulsion, and lower the analgesia requirements for ureteral stones <10 mm [54, 56]. However, treatment with calcium‐antagonists gives the highest frequency of side effects (e.g. hypotension, palpitations and headache) [57]. MET with α‐blockers was found to be beneficial, while with calcium‐antagonists was found not to be as beneficial [53]. Nonetheless, MET is recommended for ureteric stones <10 mm [52, 53].
Recently, potential new MET pharmacotherapeutics have been investigated. The selective 1A‐adrenoceptor (AR) antagonist silodosin is found to be superior to the 1A/1D‐AR antagonist tamsulosin in terms of stone expulsion rate and time to expulsion [58]. Tamsulosin is found to be significantly more effective than the calcium channel blocker, nifedipine, in relieving renal colic and facilitating stone expulsion (e.g. distal ureteral calculi); side effects were more frequently reported in the nifedipine group [59].
In conclusion, MET with α‐blockers should be considered a beneficial additive to pain treatment modalities and a potential facilitator of spontaneous stone passage for ureteral stones <10 mm. Moreover, benefits of MET with α‐blockers are best seen with stones >5 mm and stones (any size) in the distal ureter, with a nearly 7% risk of side effects from the medication [53]. However, patients elected for MET should have well‐controlled pain, no clinical evidence of sepsis, and adequate renal function. Moreover, close follow‐up is mandatory to monitor stone position, renal function, and hydronephrosis.
14.10.3 SWL
SWL is a noninvasive procedure reserved for the treatment of renal and ureteral stones (usually no larger than 2 and 1 cm, respectively). It consists of stones fragmentation through shock waves generated by a lithotripter (Figures 14.13–14.15), which allows the stone to break up with minimal collateral damage by using externally applied, focused, high‐intensity acoustic pulse.

Figure 14.13 Lithotripter used at Tenon Hospital, Paris (LITHOSKOP®, Siemens).

Figure 14.14 The principle of the first Dornier extracorporeal shock wave lithotripsy (ESWL): the shockwaves generated by an electrical spark at the first focus of an ellipsoidal mirror were reflected onto the second focus where the stones were targeted using X‐ray control.

Figure 14.15 The principle of piezo‐electric lithotripsy: a battery of piezoceramic shock generators are mounted in a hemispherical dish: the shockwaves are focused on the centre of the sphere, where the stones are targeted using ultrasound.
It may take several sessions for an optimal stone fragmentation according to the characteristics of the patient and the stone because the goal is to obtain small residual fragments susceptible for spontaneous expulsion with the urine. More than 90% of stones in adults might be suitable for SWL treatment [25, 26].
14.10.3.1 Lithotripter
Over the last two decades a lot of lithotripters of second and third generation have been developed. The first machines included a bath tub in which the patient was plunged during the treatment and usually required general anaesthesia because the crossing of shock wave through the flank was painful.
Although the progress in technology has made the new machines user‐friendly and the procedure is well tolerated, the efficacy of lithotripsy has not increased.
Lithotripters differ in the tracking system and shock‐wave–generation process.
14.10.3.2 Imaging System
Stone location can be obtained with ultrasonography, fluoroscopy, or a combination of both techniques. Indeed, lithotripter of new generation can offer both systems, but their simultaneous use is not possible.
Ultrasonography imaging system can monitor continuously during the procedure, and it is safer than fluoroscopy because it does not expose patient and staff to ionising radiation. However, it requires a highly trained operator and allows identification of stones located in the kidney and in the proximal or distal part of ureter; on the other hand, fluoroscopy is user‐friendly and allows identification of stones along the whole upper urinary with a better evaluation of stone fragmentation. However, fluoroscopy is not recommended when stones can be easily followed with ultrasonography or in children, and it is unable to visualise radiolucent calculi. In these cases or in presence of small fragments, the injection of radiographic contrast agent can aid in stone localization.
14.10.3.3 Shock Wave Generator
There are various types of shock‐wave–generation systems able to produce high‐pressure acoustic waves, discharging their energy when it gets in touch with high‐acoustic impedance index tissue (i.e. stones, bones, air). They fragment stones through complex phenomena of compression, torsion, squeezing, and cavitation.
14.10.3.3.1 Electrohydraulic Generator
In the electrohydraulic shockwave lithotripter, a high‐voltage (15–20 kV) spark discharge is applied to two opposing electrodes placed underwater at one focus (called F1) of an ellipsoid. To be correctly targeted by the spherically expanding shockwave generated on the electrode tip, the stones shall be placed at the other focus (called F2) of the ellipsoid. Generally, 500–2000 shockwave impacts at 15–20 kV are enough for disrupting 1‐cm stones, with a great variability according to the stone composition. To improve the stability and the convergence of the shockwaves, an electro‐conductive generator has been developed, using a bulb which maintain the electrode fixed and generates a more stable and convergent shockwave front.
14.10.3.3.2 Electromagnetic Generator
Electromagnetic waves are produced through a rapid displacement of two metal cylindrical plates contained into a water‐filled shock tube and separated by a thin insulating sheet. When an electrical discharge crosses one or both the conductors, a strong magnetic field moves the plate against the water and generates a pressure wave. An acoustic lens allows the shockwaves to converge towards a focal point, where the stone is supposed to be placed. Shockwave impacts can go from 3000 to 4000 at 13–17.5 kV per session and cross the patient’s body over a large skin area, causing less pain.
14.10.3.3.3 Piezoelectric Generator
The piezoelectric waves are generated through the excitement a mosaic of small, polarised, polycrystalline, ceramic elements (barium titanate) obtained with the application of a high‐voltage pulse. The rapid expansion of the piezoelectric elements, placed on the surface of a hemispherical cup, generates the shockwaves. The focus of the system is placed at the geometric centre of the spherical dish, where the stone should be placed. It is recommended not to exceed 3000 shockwave impacts per session.
14.10.3.3.4 Mechanism of Action
When shockwaves encounter the stones, they generate air bubbles organised in clusters through a cavitation phenomenon on the gas contained within the tissues, which exercise distortion forces and circumferential pressures (squeezing) on the surface of the stones, causing the disruption of the crystalline structure and the generation of fragments of 2 mm in size. Recent studies have demonstrated that to obtain a good fragmentation, the shockwaves should focus on an area larger than the stone size to take into account the stones movements caused by respiration, and the shockwave frequency should be maintained at 1–1.5 Hz [60, 61].
To improve outcomes of SWL, some introduced the extracorporeal lithotripsy endoscopically controlled by simultaneous flexible ureterorenoscopy (LECURS) [62, 63] which allows visual evaluation of the stone fragmentation. Therefore, an inadequate targeting of the stone can be immediately assessed and the focal zone can be readjusted quickly for maximal efficacy.
14.10.3.3.5 Complementary Exams and Contraindications to SWL
- Although a standard antibiotic prophylaxis is not routinely recommended, urine culture should be performed before any extracorporeal treatment, especially in case of indwelling catheter, nephrostomy tube, or infectious stone to administer the proper treatment on the basis of anti‐biogram and to avoid systemic bacterial dissemination because an active upper urinary tract infection represents a temporary contraindication to SWL.
- Coagulation tests, prothrombin time (PT), activated partial thromboplastin time (aPTT), and platelet count should be evaluated before SWL because bleeding diatheses have to be compensated for at least 24 hours before and 48 hours after treatment [64].
- Pregnancy is an absolute contraindication to SWL because of the risk of shock wave‐induced foetal lesion [65], and the potential dangerous effects of radiation exposure on the foetal development when the lithotripter adopts fluoroscopy as imaging system. Therefore, β‐hCG dosage in women of child‐bearing age is mandatory before SWL.
- Aortic or renal artery aneurysm can be damaged during SWL, and these conditions should be ruled out in patients who are elderly or vasculopathic before starting treatment [65, 66].
- Obesity or skeletal malformation can make targeting of the stones extremely difficult, and in these kind of patients, a different treatment option should be considered.
- An obstructing stone should not be routinely managed with lithotripsy as a first treatment option because drainage should be established prior to it. Routinely placement of JJ stent before SWL is not recommended because it reduces the risk of renal colic and obstruction, but it does not reduce formation of steinstrasse or infective complications and may even hamper stone clearance [67].
14.10.3.3.6 Outcome Predictive Factors
Before addressing patient with urolithiasis to SWL, it is crucial to assess the rate of treatment success, which is strictly correlated not only to the number, size, and location of the stone, but also to its hardness and its chemical composition.
Size and Number of Stones
SWL as well as endoluminal surgery can be done for the treatment of all renal stones <20 mm except for the lower pole renal calculi. Moreover, SWL is the recommended treatment option for proximal ureter stone <10 mm. It has been demonstrated that in patients with renal stones >20 mm, SWL is feasible, but the stone‐free rate at three months is significantly affected by several factors (i.e. the number of stones, a surface area > 400 mm2, and the presence of anatomical abnormalities or multiplicity of stones) [68]. Concomitant renal failure is also associated with incomplete results and increases the risk of complications [69]. Although a stone size of 20–30 mm represents the most accepted cut‐off value for considering a patient eligible for SWL, it has been proposed a maximum of 7 mm for the treatment of distal ureteral stones [70].
Lower Pole Stones
For the lower pole stones, SWL is not recommended if any of unfavourable factors for success are present – namely, shockwave‐resistant stones (calcium oxalate monohydrate, brushite, or cystine), steep infundibular‐pelvic angle, long lower pole calyx (>10 mm), and narrow infundibulum (<5 mm). However, the impact of the anatomy of the collecting system in patients with lower pole stones has not been unequivocally demonstrated, as shown by a study which found that neither the infundibulum length and width nor the infundibular‐pelvic angle significantly affected the stone‐free rate in patients with lower calyx stones [71].
Stone Density at CT Scan
Stone density at CT scan represents a major predictor of SWL success. Initially the maximum density correlated with acceptable stone fragmentation rate was comprised between 750 and 1000 HU [72]. Subsequently, a cut‐off value of 1000 HU has been suggested for admitting patients to SWL [73, 74]. Indeed, stone‐free rate reported at three months in patients with 5–20 mm stones treated with SWL were 46% and 17% in patients with stones <1000 and > 1000 HU, respectively [75]. Moreover, HU > 1000 resulted the only independent predictive factor of the presence of residual fragments after SWL [76].
Physico‐Chemical Stone Characteristics
In vitro studies showed that the efficacy of SWL depends on stone composition and its crystalline structure. The softest stones are represented by those composed of uric acid, followed by magnesium ammonium phosphates (struvite structure), calcium oxalate di‐hydrate (wheddellite structure), and carbonate apatite phosphate (dahllite structure). On the other hand, stones made of calcium hydrogen phosphate (brushite structure), calcium oxalate mono‐hydrate (whewellite structure), and cystine are the most difficult to break.
Therefore, several studies tried to predict the stone composition relying on the stone attenuation coefficient at CT scan measured as HU [77–80]. Recently, the introduction of dual‐energy CT scan has allowed defining the composition of the stone with good accuracy [81, 82]. However, the correlation between CT scan characteristics and SWL failure rate is not linear because the cystine stones have lower X‐ray attenuation coefficient than calcium oxalate di‐hydrate stones, but its resistance to SWL is greater compared to wheddelite. Moreover, despite identical chemical composition, wheddelite and whewellite stones respond very differently to shockwaves. These data suggest that not only the chemical composition but also the crystalline organisation of the stone has a relevant impact on SWL efficacy [83].
14.10.3.3.7 SWL Complication
The introduction of new lithotripters and the optimization of SWL parameters allowed reduction of side effects, which include direct tissue effects on renal parenchyma and nearby organs and obstructive problems due to residual fragments and systemic complications.
Renal and Nearby Organ Injury
The passage of shockwave front through the body has plenty of interactions with tissues and can cause different side effects in terms of severity and involved organs.
- Blood test or urinalysis alterations are often found but generally regress spontaneously without any relevant clinical impact (a transitory increase of bilirubin, lactic dehydrogenase, transaminases reveal liver injury; alterations of myoglobin and creatinine phosphokinase reveal muscle contusion; and proteinuria indicate renal parenchymal injury).
- Pain during the procedure can affect outcomes; however, simple analgesics, NSAIDS, and opioids can control pain adequately [84].
- Haematuria is the most common event after SWL but usually ends in few days and does not require any further treatment.
- Perirenal hematomas (subcapsular or intraparenchymal) are detected at ultrasound or CT scan in 15–30% of patients who undergo SWL, but it can rarely become symptomatic and require treatment (i.e. arterial embolisation or surgical evacuation) in less than 1% of the cases [85, 86].
- Microvascular injury associated with shockwave has been also suggested to have a role on long‐term effects, manifested as a vasoconstriction which could cause a decrease in glomerular filtration rate (GFR) [87, 88], diabetes mellitus – secondary to pancreatic lesions‐ and hypertension [89, 90]; however, these hypotheses remain controversial [91].
Residual Fragments
Residual fragments after SWL are often expelled spontaneously. However, if they remain inside the renal cavities, they increase the risk of new stone formation and persistent urinary tract infection.
It has been demonstrated that size is the most important predictor of stone progression and further intervention [92–94]. Therefore, if a patient remains asymptomatic and does not have associated metabolic alterations, stone fragments <4–5 mm can be followed‐up regularly to monitor disease course and avoid complications.
According to stone analysis, patient risk group, and metabolic evaluation, medical therapy should be considered to improve fragment clearance [95, 96]. Tamsulosin has been demonstrated to increase stone‐free rate and painful episodes recurrence [97], especially in patients with >10 mm stones treated with SWL [98].
According to the size, the number, the location, and the anatomy of the excretory axis, residual fragments can dislocate and cause an obstruction of the upper urinary tract and painful episodes (renal colic). A column of stone fragments accumulated in the ureter (steinstrasse) has been observed after SWL in 24% of patients with renal stone ≥2 cm and 9.5% of patients with ureteral stone >1 cm [99]. The hardness of the stone, which affects the efficacy of SWL and the size of residual fragments, was found to be the strongest predictor of steinstrasse [100]. In such cases, a drainage of renal cavities is often necessary, obtained through either the placement of JJ stent or PCN. Residual fragments need to be subsequently treated with a second SWL treatment or endourological or percutaneous procedure.
Systemic Complications
- Bacterial superinfection after SWL is uncommon but can occur especially in case of infection stones (struvite or carbonate apatite phosphate stones) because shockwave release a considerable amount of germs when they impact the stone, or in the presence of residual fragments, which can cause obstruction and acute pyelonephritis. It requires patient hospitalisation and proper measures, consisting of wide spectrum antibiotics and drainage of the excretory axis [101, 102]. To prevent this condition, urine culture is mandatory before any treatment option, and antibiotics must be administered in case of bacterial colonisation or infection stone for three to five days before SWL.
- Acute renal failure occurs only in case of bilateral SWL if residual fragments dislocate into both ureters, causing bilateral obstruction or for the establishment of acute tubulopathy, which usually regress without sequelae.
14.10.3.3.8 Factors Affecting SWL Results
To obtain satisfactory results, the expertise of the operator is essential [103], together with the effectiveness of the lithotripter, which needs to supply a wide focal width, which takes account the spontaneous respiratory movement for a good real time imaging system, and shock‐wave parameters (i.e. frequency, potency, pulse number) [104] adjustable according to patient characteristics. In particular low frequency (1 Hz) [105] and progressive increase of voltage provides better outcomes [106].
14.10.3.3.9 Follow‐Up
It is mandatory to evaluate the results of stone fragmentation and eventually the presence of residual fragments at KUB or ultrasound within one week after SWL. The patient should be advised to filter the urine to monitor spontaneous expulsion fragments. If residual stone persist, radiological imaging is recommended every three to six months during the first year and then annually.
14.10.3.4 URS (Rigid/Flexible)
Over the last two decades, the use of URS has been continuously rising thanks to the miniaturisation of technology, the development of flexible tools, enhanced optical quality, and the amelioration of stone intracorporeal fragmentation. The technique consists of accessing to upper urinary tract crossing the urethra and the bladder with an endoscopic instrument (ureterorenoscope) which allows localisation, extraction, or fragmentation the stone and removal of residual fragments.
The optimal outcome of URS is the relief of ureteral or renal collecting system obstruction and stone extraction or disruption with complete removal of residual fragments. When these conditions are satisfied, the patient is considered stone free.
URS and stone fragmentation or retrieval can be performed using a rigid or semi‐rigid or flexible ureterorenoscope, according to the location of the stone (Figure 14.16).

Figure 14.16 Rigid or flexible fibre‐optic ureteroscopes may be passed right up into the renal calices.
Usually, when the stone is located along the ureter, especially under iliac vessels, a rigid or semi‐rigid ureterorenoscope is preferred because it straightens the ureter and allows it to easily ascend the ureter as well as help maintain to fix the stone in front of the instrument during lithotripsy.
However, the stiffness of a rigid or semi‐rigid instrument becomes a disadvantage when the stone is located in the kidney and an exploration of all the renal cavities is required. For this reason, the flexible ureterorenoscope has been developed over the last two decades, allowing an endoscopic retrograde treatment in almost all the cases [107, 108].
14.10.3.4.1 Preoperative Work‐Up
- An image depicting the size, number, and location of stone (ultrasound or KUB), but also the anatomy of the upper urinary tract (uro‐CT scan, magnetic resonance urography (uro‐RM), anterograde or retrograde) is mandatory before surgery to evaluate the feasibility and safety of a retrograde stone manipulation.
- Urine culture has to be negative before URS, and antibiotics prophylaxis should be administered. The presence of obstructive pyonephrosis forces the procedure to be postponed and requires the drainage of the obstructed excretory axis through the placement of JJ stent or PCN [42, 46].
14.10.3.4.2 Anaesthesia
General or loco‐regional anaesthesia can be used according to surgeon and anaesthetist preference, although for stones located in the kidney or the proximal part of the ureter, a general anaesthesia with curarisation of the patient may help the progression of the ureterorenoscope along the ureter releasing ureteral smooth muscle fibres [109]. Moreover, with general anaesthesia, a limited period of apnoea is feasible and prevents kidney movement due to respiratory acts which may make the stone fragmentation more difficult.
14.10.3.4.3 Positioning of the Patient
The lithotomy position is used to offer the greatest mobility to the operator. In the past years the leg ipsilateral to the stone was maintained horizontal to enhance the release of psoas muscle and the passage of the ureterorenoscope, although the decrease of the size (7–9 Ch) and the increase of the flexibility of endoscopic tools has actually made this procedure inessential.
14.10.3.4.4 Materials Used and Technical Procedure
- Cystoscope: 22–25 Ch calibre (1 Charrière = 0.33 mm), with optics 12–30° for exploring the bladder and identifying ureteral orifice.
- Ureteral dilation system: olivary dilator, expansion plugs, and probes balloon (maximum 8 atm) are used to dilate the ureter, although this procedure is rarely required due to small diameter of the last generation tools. Moreover, to force the passage of the instruments into the ureter is not recommended because these may cause ureteral lesion and development of ureteral stricture. If ureteral access is not possible, a valid alternative is represented by the insertion of a JJ stent followed by URS after 7–14 days.
- Guidewire: 0.035 or 0.038 in. diameter 150 cm‐long is used for cannulating ureteral orifice, having access to renal collecting system and allowing passage of stents, catheters, and ureterorenoscopes. One ‘safety’ and one ‘working’ wire should be used.
- Ureteral catheter: 5–7 Ch open tip, for retrograde pyelography, for guide‐wire positioning or for obtaining urine sample for culture at the beginning of the procedure.
- Irrigation system: saline is used to clean the renal cavities during the whole procedure. An adequate flow allows a good view, which is essential for achieving an optimal outcome. The flow depends mainly on the size of the ureterorenoscope and the presence of an instrument into the working channel. Indeed, when a 2.4 Ch instrument is placed into the working channel, the flow provided by a bag of saline placed at 60 cm height decreases from 40 to 10 ml min−1 [110]. For this reason, irrigation pump system has been developed to provide an adequate flow during all the phases of the surgery [111].
- Video Camera: it allows operative assistance and active training. Since 2006, the introduction of a miniaturised digital system (CMOS and CCD sensors), incorporated within the distal part of the instrument, has improved the quality of view. It has been recently demonstrated that digital ureterorenoscope significantly reduced operative time compared to fibre optics ureterorenoscope by 20–25% [112]
- Rigid or semi‐rigid ureterorenoscope: 7.5–12 Ch, the size usually decreases progressively moving towards the tip. The optical system adopted makes the difference between rigid and semi‐rigid instrument. Indeed, the use of fibre optic (semi‐rigid) rather than lens (rigid) gives a certain flexibility to the ureterorenoscope. It is generally used for the treatment of ureteral stones.
- Flexible ureterorenoscope: 7.4–9 Ch, as well as the rigid instrument, the tip is thinner than the proximal part. Moreover, the tip has an active deflection of 180° either ventral or dorsal. Since 2001, the deflection degree has been increased at 270°, generally towards the ventral side, to gain access to small calices with a steep infundibular‐pelvic angle. As well as the rigid instrument, it has a working channel which allows the placement of operative tools (i.e. laser or basket), but it causes a reduction of the inflow. The quality and the number of fibre optics contained in the instrument for transmitting the light source and especially for acquiring the images determine the quality of view (Figures 14.17 and 14.18).
- Stone fragmentation tools: Holmium‐YAG laser has become the standard for intracorporeal lithotripsy during ureterorenoscopy, although other methods still exist (i.e. pneumatic or ultrasonic system). Its wavelength (2140 nm) allows a strong absorption of the water and a tissue penetration of only 0.4 mm. It acts on the energy of light beam is delivered in a pulsatile manner, and it is transformed in a thermo‐mechanical action able to fragment any type of stones [113]. Each pulse is defined by a specific energy (Joule) and frequency (Hz). The power, expressed in Watt, is the product of energy multiplied by frequency. It is possible to modulate the laser effect changing the values of these parameters. Usually laser fibres adopted for lithotripsy allow a maximum power of 10 W. The diameter of laser fibres goes to 150–365 um, and they have to be managed carefully because they are very fragile and may break inside the ureterorenoscope, especially when the tip is strongly deflected, causing a damage of the instrument.

Figure 14.17 Different ureterorenoscope available on the market: digital (URF‐V, URF‐V2 – Olympus – and Flex‐XC – Stortz) and fibre optic (URF‐P5 – Olympus –, Flex‐X2 – Stortz – and URF‐P6 – Olympus).

Figure 14.18 The ‘semi‐flexible’ URF‐V2 and URF‐P6 (Olympus) versus the flexible URF‐V and URF‐P5 (Olympus).
Though largely replaced by the advancement in laser technology: some centres still use:
- Ultrasonic probe. A toothed cylinder is made to oscillate at ultrasonic frequency (Figure 14.19). It grinds the surface of the stone into fine powder, which is sucked away in a current of irrigant.
- Electrohydraulic lithotriptor. A pair of parallel or concentric electrodes connected to a condenser emits a spark in contact with the stone (Figure 14.20).
- Jackhammer. A miniature road‐drill is powered by compressed air. Of all the gadgets, this appears to be the most effective and versatile. It is also one of the least costly of these expensive implements (Figure 14.21).
- Extraction tools. Graspers or baskets of different size (1.2–3.0 Ch) and shape are placed through the ureterorenoscope working channel and used for residual fragments extraction. The most common used baskets are made of nitinol 1.9 Ch because they guarantee resistance and does not lose flexibility [114]. Tipped baskets are routinely used in the ureter; however, they have a risk of causing ureteric damage. Tipless baskets are used in the renal pelvis and calyces.
- Imaging system. Fluoroscopy is essential during any procedure because it allows to follow the progression of endoscopic tools inside the ureter and to evaluate the anatomy of the excretory axis with medium contrast injection before the beginning of the procedure and the presence of stones or residual fragments
- Ureteral access sheath. 9.5–11.5 Ch, 10–12 Ch, 11–13 Ch and 12–14 Ch, it is placed in the ureter with the aid of fluoroscopy over the guidewire till the ureteropelvic junction, but it can reach the renal cavities or be stopped along the ureter according to the urologist’s preference. It protects the ureterorenoscope and facilitates its positioning into the upper urinary tract, especially when the surgeon has to go back into the renal cavities repeatedly to remove residual stone fragments after laser lithotripsy. Finally, it guarantees a constant backflow during all the procedure and avoids elevated pressure in the kidney, reducing the risk of intraparenchymal reflux and thereby decreasing infectious complications. However, it should be placed gently because it can cause ureteral lesion (Figure 14.22) [63].
- Ureteral drainage. Ureteral catheter or JJ stent (of different size, shape and materials) (Figure 14.23) may be placed at the end of the endoscopic procedure to guarantee the drainage of renal cavities and avoid postoperative complications, especially after a long procedure, in presence of residual fragments or ureteral lesion, and when URS is used [115]. It should be removed within 1–2 weeks. In case of uncomplicated URS with no residual fragments, stenting should be avoided because it is found to be associated with higher postoperative morbidity [116–118]. As well as for SWL, routine stenting is not recommended before URS, although it can facilitate ureteroscopic management of stones and potentially improve the stone‐free rate [119].
- Jackhammer. A miniature road‐drill is powered by compressed air. Of all the gadgets, this appears to be the most effective and versatile. It is also one of the least costly of these expensive implements (Figure 14.21).

Figure 14.19 Ultrasonic lithotripsy: a toothed cylinder is made to oscillate at ultrasonic frequency to grind the stone to a powder which is aspirated in a current of irrigant.

Figure 14.20 Electrohydraulic lithotripsy: concentric or parallel electrodes emit a spark which shatters the stone.

Figure 14.21 The Swiss lithoclast: on the principle of the jackhammer probes of varying flexibility may be passed up the ureter or into the renal pelvis to break up a stone.

Figure 14.22 Different ureteral access sheath available on the market and the related radio‐opacity.

Figure 14.23 If a double‐J stent is left in the ureter, it becomes dilated and allows fragments of stone to pass down.
14.10.3.4.5 URS Outcome
Major technological progress achieved for new generation flexible ureterorenoscopes allows to offer higher treatment success than their predecessors [120]. The stone‐free rate following URS for the treatment of ureteral stones varies from 80 to 97% depending on the location and size of the stone [52, 121–123]. URS is the best treatment option for all ureteral stones, except for the proximal ureter stones <10 mm, where SWL shows higher stone‐free rate (89 vs. 84%, respectively).
Flexible URS is equally recommended along with SWL for the treatment of all renal stones <20 mm because stone‐free rate are comparable [124, 125] and can achieve up to 90% [126].
However, for 10–20 mm lower pole stone ureterorenoscopy becomes the first treatment option [127, 128], especially when there are unfavourable factors for SWL success. It is recommended to relocate the lower pole stone into the superior calyx or into the renal pelvis for laser lithotripsy [129], to make the stone fragmentation easier to perform, and to reduce the risk that the laser fibre can fail under a tight bending and damage the ureterorenoscope.
Moreover, flexible URS for the treatment of renal stones >2 cm is feasible and has a high stone‐free rates and low complication rates [130].
14.10.3.4.6 URS Complication
The overall complication rate after URS is up to 10% [52, 131]. The miniaturisation of endoscopic tools and improvement of technology has decreased the incidence of complications related to ureterorenoscopy. Because the major cause of complications is the trauma caused by the surgery, they occur more often after rigid or semi‐rigid URS than after flexible URS.
Early Complications
They may happen during the procedure or immediately after.
They include:
- Ureteral false passage and mucosal injury: no need for specific manoeuvres, the ureterorenoscopy can be continued and a JJ stent should be placed afterwards.
- Ureteral perforation: suspected in presence of trauma or bleeding and diagnosed by retrograde pyelography, which shows medium contrast extravasation; it requires the interruption of the procedure to avoid the development of a urinoma and the placement of a JJ stent for three to four weeks to promote the healing process.
- Ureteral avulsion: it represents the most feared complication. It occurs in 0.2% of the cases usually at the pelviureteric junction or at the pre‐vesical tract, which are the most fragile point of the ureter. It requires a reconstructive surgical intervention (i.e. ureteral reimplantation or ureter‐ileum interposition according to the location of the avulsion). The surgeons must prevent it by avoiding removal of large stone fragments from the renal cavities and stopping the extraction of the stone fragments when an excessive resistance is perceived.
- Renal colic and fever: back pain and fever usually regress spontaneously within 24–48 hours or with the use of analgesic and antibiotics, respectively. If the urine was sterile and an antibiotic prophylaxis was given before the surgery, serious infectious complications are uncommon.
Late Complications
The incidence of late complications is 0.5%. They include:
- Ureteral stricture: caused by ureteral trauma, it can be avoided placing a JJ stent after the surgery. A CT scan with delayed images, during follow‐up is recommended not only to evaluate the presence of residual fragments but also to exclude this condition. It can require ureteral balloon dilation, endoscopic ureterotomy or laparoscopic or open surgery.
- Vesicoureteral reflux: when occurs, it usually involves the distal part of the ureter only and it can be managed conservatively.
14.10.3.5 Percutaneous Nephrolithotomy
The first description of PCN tube to obtain kidney drainage was described by Goodwin and associates in 1955. Then in 1976, Fernstrom and Johannson were the first to report the placement of percutaneous access with the planning of kidney stone removal. Today, the rates of percutaneous nephrolithotomy (PCNL) performed for kidney stone treatment is between 3 and 5%, and this procedure is mainly reserved for large stone burden.
Having inserted a nephrostomy tube over a guidewire, the track is dilated until a 28 Ch sheath can be passed (Figure 14.24). This is large enough to admit telescopes, forceps, and lithotriptors.

Figure 14.24 Percutaneous nephrolithotomy. Tubes of increasing diameter are introduced over a guidewire until one can be passed large enough for the introduction of a nephroscope.
14.10.3.5.1 Indications
With the technology emergence of flexible ureteroscopy (FURS) in the last decades, which can be effective even for stone larger than 2 cm and staghorn calculi, PCNL is likely to decrease and remain principally in the specialised centres.
There are still indications to perform a PCNL including the following: stone larger than 2 cm; inferior polar stone larger than 1.5 cm; failed FURS for the treatment of calyceal diverticula calculi; partial or complete staghorn calculi; and some cases of ureteral calculi [25, 26].
14.10.3.5.2 Contraindications
During the initial evaluation, the urologist should be prepared to identify the PCNL contraindications, such as uncorrected coagulopathy, active and untreated urinary tract infection or bacteriuria, nontreated high blood pressure, intrarenal vascular malformations, and a complex staghorn which requires more than two percutaneous tracts (relative contraindication). Severe obesity and severe skeletal malformations are not contraindications; nevertheless the technique and equipment should be adapted [25, 26, 132, 133].
14.10.3.5.3 PCNL Surgery
Patient Anaesthesia and Antimicrobials
PCNL is usually performed under general anaesthesia. Perioperative antimicrobial prophylaxis for all cases of PCNL is recommended. Local protocol should dictate the type of antibiotic. Duration of treatment should be less than 24 hours, and a short course of antimicrobials at the time of tube removal can also be considered. When there is a preoperative positive culture, appropriately directed antimicrobials should be administered for 7–10 days, particularly, when struvite infected stones are suspected [25, 26].
Patient Positioning
The first approach to PCNL initially described in 1955 was the prone position. When prone position is performed, the initial step is retrograde placement of a ureteral catheter with fluoroscopic guidance. A ureteral occlusion balloon catheter can be used to prevent stone migration into the ureter during the surgery. It allows more cavities distention during the pyelography before the puncture, but there is also more chance of calyceal rupture and contrast extravasation with deterioration of vision for getting the access. Furthermore, there is a risk of ureteral rupture if the balloon is not carefully inflated in the renal pelvis. Depending on the surgeon’s preference, the catheter can be placed directly in the prone position with a flexible cystoscope or in lithotomy position with subsequent prone repositioning.
Completely supine position PCNL has also been reported in 1987, as an alternative to the prone position. Many have added variations of this position, including supine with the ipsilateral side elevated and supine with ipsilateral side elevated combined to asymmetric lithotomy position. The advantage of combined approach is the opportunity to operate simultaneously for URS and percutaneous surgery (Figure 14.25). This could be especially helpful in complex renal calculi or large proximal ureteral stones. It could prevent the need to do a second puncture with all the associated risk and reduce the operative time. Furthermore, this approach is recommended for patients with spinal deformities, pulmonary pathologies, ASA 3, and who are morbidly obese.

Figure 14.25 Positioning for combined intrarenal surgery, Galdakao modified Valdivia position.
A less commonly applied method is the lateral decubitus position, which could be valuable in patients who are obese or have spinal deformities. Moreover, providing access to the anterior and posterior calyces.
Renal Calculi Tracking
The principal two methods used for stone localization into the collecting system are ultrasound and fluoroscopy. The use of CT scan or MRI is uncommon and only reserved for some complex cases.
Ultrasound guidance (3.5 or 5‐MHz transducer) has the advantages of having no radiation, evaluation of the renal parenchyma aspect and thickness, and greater assurance that there is no essential organ on the future percutaneous access. Retrograde indigo‐carmine instillation can be used to confirm the accurate site of the needle into the collecting system.
Fluoroscopic guidance is usually used for gaining access to the collecting system. Retrograde contrast filling is used to provide upper tract urinary system anatomy and stones location within the pelvis and calyces. Air into the cavities could also assist in locating the posterior calyces while the patient is in a prone position.
Furthermore, combining these techniques is also an excellent and safety approach.
Access into the Collecting System
Once the urologist has chosen the appropriate calyx, the needle (18 G) is advanced under guidance into the urinary cavities. During the puncture, the surgeon’s forearm should be stabilised on the patient’s back to obtain a better movement control and security. The needle should be inserted back to the posterior axillary line and the shorter cortico‐papillary distance feasible to reduce the cortical haemorrhage risk (Figure 14.26). Afterwards, a 0.038‐in. guidewire is placed within the pelvis and ordinarily down the ureter. Subsequently, an extra‐stiff guidewire may be inserted, and the dilation of the tract is made.

Figure 14.26 Access in percutaneous surgery, prone position.
There are some techniques to provide a good tract dilation. Alken’s rigid dilators are a series of enlarging coaxial metal steel rods passing over a guide rod and are remarkably effective principally, although there is perirenal scarring. Nevertheless, their rigidity can also cause incommensurable damage. Amplatz semi‐rigid plastic dilators are supposed to be less traumatic and are passed one after the other, not coaxially like Alken’s dilators. Another dilator is the balloon catheter dilator. This is currently the most used dilator. These benefits are being able to simplify the introduction in urinary cavities with only one entry and be more effective in case of a hyper‐mobile kidney. Although, it could be less efficient than rigid or plastic dilators while there is perirenal fibrosis. Once the dilation is created, the working sheath is positioned with fluoroscopic guidance (Figure 14.15).
Intracorporeal Lithotripsy
Once the nephroscope is introduced into the cavities, there are different ways to extract the stones. The stones smaller than 1 cm are usually removed immediately in one piece with a basket or grasper. Calculi larger than 1 cm require fragmentation first. Many types of lithotripters are used during a PCNL. The ballistic lithotripsy uses energy generated by the movement of a projectile that transfers energy to an object. Ultrasonic lithotripsy applies electrical energy to excite a piezoceramic plate. The plate resonates at a particular frequency and generates ultrasonic waves with transmitted energy. Including with this lithotripter is a suction mechanism that can remove continuously small particles during the fragmentation. The combined ballistic and ultrasonic devices are significantly better for the stone clearance. It may be activated separately or simultaneously, and the surgeon has the benefits of each instrument. After a maximal stone removal with the rigid nephroscope, exploring the renal cavities and the proximal ureter with a flexible nephroscope to ensure there is no residual stone fragment is advised. Otherwise, these fragments can be repositioned or fragmented with Holmium: YAG laser.
In large stones, combination PCNL and FURS can be done (Figures 14.25 and 14.27).

Figure 14.27 Fluoroscopy during combined intrarenal surgery illustrates working sheath (30 Fr) and retrograde flexible ureteroscope.
Nephrostomy Drainage after the Procedure
At the end of the surgery, a nephrostomy tube is inserted (12–24fr), and the adequate placement is confirmed with fluoroscopy and pyelography. Day one postoperatively, a CT scan or abdominal X‐ray is performed to rule out any residual significant fragments. When there are significant fragments, a second look is recommended 48–72 hours after the PCNL. However, where imagery confirms there are no stones and urine are clear, the nephrostomy may be removed 24–72 hours later with a clamp test just before. The possibly externalised tubes are Malecot catheter, Malecot re‐entry, the balloon catheter (Foley and Councill), Cope catheter, and circle nephrostomy tube.
Some authors report tubeless percutaneous procedure with a ureteral stent. It seems to have an advantage of reducing narcotic use and decrease the length of hospitalisation, and the complications appear similar. The nephrostomy, on the other hand, provides more security and rapid access if the patient requires a second look. The selected patients for tubeless have to be operated with care in a high volume centre. Totally tubeless has also been reported, but there appears to be significant concern with the theoretical risk of haemorrhage from the tract following PCNL.
Hospitalisation and Recovery
Hospitalisation may vary from one to five days. Patient’s occupation should allow two weeks following the surgery unless this is a hard physical work. Sport activities could be reintroduced after four weeks.
Complications
The overall complication rate for a PCNL varies from 10 to 25%. The urologist aspiring to practice this procedure has to be aware of the potential complications and be conscious of how controlled the situation to avoid significant morbidity.
Patient Positioning A meticulous verification of every pressure point has to be accomplished by the anaesthesiologist and urologist because nerve compression, stretch injury, ocular or facial pressure wound, and rhabdomyolysis have been described in the prone position [134, 135].
Haemorrhage Acute Acute haemorrhage is the most prevalent complication. Transfusion rate varies from 6 to 24% and depend on several considerations. Intraoperative haemorrhage necessitates ending of the procedure and placement of a nephrostomy tube to allow the formation of a clot into the renal cavities. A balloon catheter could reinforce the local pressure and permit more haemostasis with a small traction. A venous bleeding could be controlled most of the time with this method. If the bleeding originates from the tract of the working sheath after its removal and is refractory to the techniques described previously, a Kaye nephrostomy tamponade balloon can be used. If the haemorrhage cannot be controlled, a supra‐selective angio‐embolisation must be required. Nephrectomy is rarely needed [132, 136].
Delayed Less than 1% of PCNL are complicated with a delayed haemorrhage and are usually secondary to a pseudo‐aneurysm or arteriovenous fistulas. Delayed haemorrhage occurs mostly between two and seven days after the surgery. The diagnosis is made with angiography, and the treatment is selective angio‐embolisation with high success rate (>90%) [137].
Irrigation Absorption Major fluid absorption is rare (<1%). A procedure more than two hours, venous laceration, and more than 30 l of irrigation are the risk factors. While complex and prolonged case is planned, the surgeon should recommend the in and out irrigation monitoring [132].
Renal Urinary Collecting System Laceration Renal pelvic perforations are usually identified intraoperatively (3–6%). It is recommended to abort the surgery and insert a ureteral stent catheter and a nephrostomy tube. A nephrostogram could be done after two to seven days, depending on the severity, and maintaining the ureteral stent for some weeks is more suitable. Antimicrobial prophylaxis depends on the surgeon’s preference, the risk of urinary extravasation, or urinoma formation [138].
Visceral Injury
Pleural Pleural injuries are usually associated with punctures above the 11th and 12th ribs and remain rare complications (<0.5%). Hydrothorax or pneumothorax can be treated most of the time with a small‐calibre tube depending on the clinical indication. Rarely a large‐bore thoracostomy is needed. Urinothorax (nephropleural fistula) is a persistent communication between renal cavities and thoracic cavity. Drainage of each cavity is recommended with antimicrobial prophylaxis [139].
Colon Colon injury occurs less than 1% of PCNL in the prone position. Left lower pole punctures are the principal zone associated with colon injury. Intraoperative detection is easier to manage; the nephrostomy tube should be pulled into the colon, and a retrograde ureteral stent catheter or a new nephrostomy should be inserted. In addition, broad‐spectrum antibiotics and no oral intake for a couple of days are recommended. The principle of care is a separate drainage of the urinary system and digestive system. Open surgery is rarely required unless intraperitoneal injury with sepsis is diagnosed. Colonic injury can be avoided when the puncture is performed with ultrasound guidance [140].
Others Duodenal, jejunal, gallbladder, splenic, and hepatic injuries have all been reported in few cases, and the clinical presentation and seriousness of the injury would decide the future management.
Postoperative Fever and Sepsis Fever following PCNL can develop in 15–20% of patients. However, sepsis occurred in less than 1% of patients. All infected stones should receive preoperative antibiotic treatment for seven days. This patient should also have an intraoperative pelvis urine culture and a stone culture because frequently the bacteria identified in the preoperative urinary culture is different from the pelvis and stone cultures. Absolutely no patient should be operated with a preoperative positive culture [141, 142].
Parenchyma Injury Significance All the studies to evaluate parenchymal injuries after PCNL have demonstrated that the loss of renal parenchyma is less than 1%. Moreover, no significant difference for kidney injuries has been associated between the miniperc (11 Fr) or standard PCNL (30 Fr) [143, 144].
MINIPERC
The mini‐PCNL or miniperc was initially developed for the treatment of children with complex stones, and there are great efficiency and less morbidity. The advantage is the working sheath of 13 Fr–20 Fr. Indications expansion have been attempted for adult community with mainly stones less than 2 cm, but with all the advancement in the retrograde intrarenal surgery with flexible URS, the field for miniperc is still limited [145–148].
14.10.3.6 Laparoscopy, Robotic, and Open Stone Surgery
Historically, open surgery was the treatment of choice for symptomatic upper urinary tract calculi. However, the technology advancement in minimally invasive endourology procedures (i.e. PCNL and FURS) have considerably reduced the place for open and laparoscopic stone surgery, which is now used in less than 1% of cases.
The FURS, PCNL, or combined surgery have advantages in comparison to laparoscopic or open surgery with lower morbidity and similar overall stone‐free rate. Invasive procedures continue to be an option for some specific conditions, including treatment failure with endoscopic procedures, intrarenal anatomical abnormalities (i.e. infundibular stenosis, calyceal diverticula), pyeloplasty with pyelolithotomy, and stones in a nonfunctioning polar or nonfunctioning kidney.
Currently, for rare cases requiring more invasive surgery, laparoscopic, or robotic approaches should be recommended first, and it may be advisable to refer the patients at an experienced center. Chosen methods may change depending on the location and size of the stone.
14.10.3.6.1 Kidney Calculi
The possible operative procedures for complex kidney calculi include the following: anatrophic or radial nephrolithotomy, simple or extended pyelolithotomy with or without concomitant pyeloplasty, and partial or polar or simple nephrectomy.
The nephrolithotomy is principally reserved for cases that have failed with endoscopic procedures. The most common is a complex diverticula or a long narrow infundibulum. Throughout the laparoscopic intervention, the stone is localised, and a nephrotomy is performed to completed the stone extraction. Intraoperative ultrasound has been described to find stone location and identified the avascular area in the renal parenchyma. A careful cavities closure and haemostasis is mandatory. Upper tract urinary drainage is dependent on the surgeon’s preference, and a perinephric drain should be placed for a few days.
Pyelolithotomy is used most of the time through concomitant laparoscopic pyeloplasty surgery. Concurrently, a flexible cystoscope is entered in the pyelocalyceal cavities, and a basket removal of the stone is completed. Some urologists prefer first to do a minor pyelotomy sufficient to enter the flexible cystoscope and to get irrigation in the cavities. Following the stone removal, they complete the dismembered pyeloplasty.
Partial, polar, or simple nephrectomy are reserved for complex cases with partial and total nonfunctioning kidney or recurrent infection or abscess, as in xanthogranulomatous pyelonephritis. These patients need a definitive treatment for their pathology [25, 26, 132].
14.10.3.6.2 Ureteral Calculi
Open and laparoscopic surgery for ureteral stone remain a seldom procedure. Invasive surgeries are reserved in situations such as failed previous endourology procedures or SWL, complex stone burden, and extended stenosis that need to be corrected through the same procedure. Laparoscopic ureterolithotomy has been used successfully with lower than 2% conversion rate to open surgery.
A longitudinal ureterotomy is performed at the ureteral stone level, and the stone is removed. Then, a ureteral stent is inserted in the course of laparoscopic surgery while achievable. The authors advise to insert a ureteral stent because there is frequently inflammation at the level of the stone, and the mucosa is crumbly during the closure. A ureteral stent may contribute to decrease the formation of ureteral stenosis. Furthermore, to monitor any urinary leak, a retroperitoneal drainage deprived of suction could be left in place in postoperative period, depending on the surgeon’s preference. A follow‐up imaging with ultrasound or technetium‐99 m‐mercaptoacetyltriglycine (MAG3) diuretic scan is preferable to exclude a ureteral stenosis formation during follow‐up [149, 150].
In summary, with all technology and equipment advancement, there are only seldom indications regarding laparoscopy and open surgery as first‐line treatment for kidney and ureteral calculi. Surgeons should never hesitate to refer patients in a specialised endourology center if FURS and PCNL are not feasible, which are now the first‐line modalities before further invasive procedures.
14.10.3.6.3 Chemolytic Dissolution for Stones
No effective and safe dissolution therapies (chemolysis) exist for calcium‐containing stones [151, 152]. Stones that can be dissolved include uric acid, cysteine, and struvite calculi. Chemolysis may be achieved by agents given orally or intravenously or by instillation of chemolytics into the collecting system. Dissolution of stones may be divided into primary and secondary chemolysis, referring to whether chemolytic agents are used as initial therapy to dissolve stones, or secondarily in patients with residual fragments after some other primary therapy.
Oral Chemolysis
Although oral chemolysis anecdotally have been described for cystine [153] and struvite calculi [154], uric acid stones are the only stones that effectively can be dissolved by oral chemolysis. Treatment consists of systemic alkalinisation in the form of sodium bicarbonate (650–1000 mg 3–4 times a day) or potassium citrate (15–30 mEq 3–4 times a day) [155, 156]. Complete dissolution rates of 62.5% have been achieved; however, such a high success rate demands prolonged therapy (three to six months), and thus is only an acceptable option in nonobstructing calculi [157, 158]. Success is related to stone size as well as patient compliance. An important part of compliance is patient self‐monitoring to verify that the urinary pH increases [159]. Goals of therapy is to achieve a urine pH between 6.0 and 7.0. Below pH of 6.0 will form uric acid stones, while pH above 7.0 will form calcium phosphate stones. Intravenous alkalinisation is more effective than oral, but it requires hospitalisation. Larger stones demands longer therapy, and in these, cases SWL may increase treatment efficacy by increasing stone surface area [160].
Instillation of Chemolytic Agents
Instillation of chemolytic agents may be done a retrograde, percutaneous antegrade, or in a combined approach. Antibiotics (i.e. prophylactic or culture‐specific) should be maintained throughout the instillation period. Intrapelvic pressure should be monitored (<25–30 mm H2O) to avoid pyelovenous and pyelolymphatic backflow that may result in septic or agent‐specific toxic complications and alterations in serum chemistries [151, 161]. Preferably, inflow and outflow is achieved through separate catheters or channels. The safest way of controlling inflow and outflow is through two nephrostomy tubes. Perirenal extravasation should be excluded by contrast studies. The major disadvantage of the retrograde approach is outflow limitation, resulting in high intrarenal pressures with potential harmful effects. Techniques to overcome this have been described [162–164]. However, in case of large stone burden that demands prolonged chemolysis, the antegrade approach seems most ideal and also most comfortable for the patient.
Dissolution of uric acid stones can be achieved by irrigation of alkaline solutions (Table 14.5) [25]. Usually prolonged irrigation is not needed.
Table 14.5 Dissolution therapy of various stones.
| Stone composition | Irrigation solution | Comments |
| Uric acid | Tham – 0.3 mol l−1 – pH 8.5 Tham – 0.6 mol l−1 – pH 9.0 | Unless a nephrostomy is present, oral chemolysis should be tried first. |
| Struvite Carbonate apatite | Hemiacidrin 10% – pH 3.5–4 Suby’s G | Usually used as secondary chemolysis. Hypermagnesaemia may occur. |
| Cystine | Tham – 0.3 mol l−1 – pH 8.5 Tham – 0.6 mol l−1 – pH 9.0 N‐acetylcysteine (200 mg l−1) | Prolonged irrigation is needed. Usually used as secondary chemolysis. Tham and N‐acetylcysteine can be used in combination. |
Chemolytic dissolution of cystine stones may be done with highly alkaline solutions (THAM or THAM‐E), the chelating solution N‐acetylcysteine, or preferably, the two types of solutions in combination creating a synergistic effect [159, 165] (Table 14.5).
The solubility of struvite is markedly increased at a pH < 5.5, which may be achieved by irrigation of Suby’s solution or Hemiacidrin (Renacidin) (Table 14.5). Fatal infectious complications have been reported using Renacidin [166, 167], and absorption of magnesium may result in hypermagnesaemia with potential cardiovascular hazards. The safety aspects of installation therapy consisting of antibiotics and monitoring of pressure and serum chemistries including serum magnesium, therefore, should be strictly observed.
Instillation therapy for cystine and struvite stones are normally performed as secondary chemolysis for residual fragments after SWL, PCNL, or URS because chemolysis monotherapy of most of these stones will require unacceptable prolonged irrigation [151, 162, 165, 168].
14.10.3.6.4 Stones in the Bladder
The introduction of the lithotrite 150 years ago may be said to have started modern instrumental urology. The best instruments are still the oldest ones, not forged, but cut from a solid block of steel. The skill of using this historic instrument has now been bypassed by the modern lithotriptors which can be passed down a resectoscope sheath to fragment a calculus in the bladder and the fragments evacuated with an Ellik evacuator. The Mauermayer punch is a most useful device for breaking up residual small fragments.
In boys and in patients with very large stones (Figure 14.28a–c), it is safer to remove them through a formal open cystotomy because the passage of urethral instruments is apt to lead to stricture.


Figure 14.28 (a) Ultrasound showing a bladder stone; (b and c) corresponding scout and computed tomography (CT) scan.
Calyceal Diverticular Stones
A calyceal diverticulum (CD) is presumed to be congenital. The have been defined as a nonsecretory transitional urothelium‐lined cavity within the renal parenchyma. Usually communicating with the collecting system (i.e. calyx and rarely renal pelvis) through a narrow diverticular isthmus or ‘neck’ (Figure 14.29). The theory is that small divisions of the ureteral bud fail to degenerate, resulting in a CD. Alternatively, some propose that CD are an acquired condition, secondary to sequelae of vesicoureteric reflux, rupture of a simple cyst, or fibrosing infundibular stenosis.

Figure 14.29 Computed tomography (CT) scan of a calyceal diverticulum containing a stone.
A rare disease with an unknown incidence; however, it is reported in about 3% of IVP investigations, occurring bilaterally in nearly 3% of patients (REF). Patients usually present with either haematuria, pain, or sepsis and form stones in up to 50% of cases.
Stones are thought to be due to either urinary stasis, whereby urine fills the cavity, and with a narrow neck, stagnates, precipitating calculi crystals. However, an underlying metabolic abnormality for stone formation is seen in over 50%.
There has been no consensus on either classifying CD or on how best to manage them. Various treatment modalities have been proposed from conservative treatment, SWL, PCNL, URS, and partial or total nephrectomy. However, management tends to be an upward trend from the less invasive to the more invasive.
Stone Management in Urinary Diversions
Patients with urinary diversions are at increased risk of upper urinary tract stones as well as calculi within the diversion segment [169]. Both medical and surgical management of stone disease related to urinary diversions are often challenging.
Risk Factors for Stone Formation
Frequency of stone formation in different types of urinary diversions ranges from 2 to 27% depending on the type of diversion, with highest frequency reported in the Kock pouch [142]. The stone disease may be classified as either infectious or metabolic.
Infectious Causes Rate of colonisation with bacteria ranges from 14 to 96% in different series depending on type of conduit or reservoir [170, 171]. Stone formation in the diversion segment is usually related to colonisation with urease‐producing organisms (e.g. Proteus and Providencia spp.), resulting in struvite and carbonate apatite crystallisation. Presence of foreign bodies (e.g. staples and nonabsorbable sutures) may act as a nidus for stone formation, which usually recurs unless the nidus is removed at time of stone removal [172]. Reflux of urine and mucus may colonise the upper tract, resulting in formation of infection stones in the upper urinary tract as well. Stomal stenosis and stenosis of the uretero‐intestinal anastomosis result in stasis of urine, which predisposes to infection and subsequent stone formation.
Metabolic Causes Metabolic risk factors for stone formation may be more or less prominent depending on which segment of the intestine that is used for the conduit or reservoir. Usage of long segments of ileum may lead to intestinal fat‐malabsorption and subsequently enteric hyperoxaluria, increasing risk of calcium oxalate (CaOx) kidney stone formation [173]. Patients with continent reservoirs are at increased risk of chronic diarrhoea depending on the length of ileum resected [169]. Chronic diarrhoea leads to gastrointestinal losses of bicarbonate, resulting in acid urine with increased risk of uric acid stone formation. Furthermore, exclusion of ileal and colonic bowel segments may result in hyperchloremic metabolic acidosis: When colonic or ileal segments are exposed to urine, ionised ammonium and chloride (Cl−) are reabsorbed by the mucosa [174–176]. Mediated by a sodium–hydrogen antiport, ammonium absorption occurs in exchange of sodium. The exchange of ammonium for H+ in turn is coupled with the exchange of bicarbonate for Cl−. Furthermore; ionised ammonium may be also absorbed into the blood through potassium channels, resulting in potential bicarbonate and potassium losses [176]. The resulting systemic acid load leads to hypocitraturia and hypercalciuria, which is known to increase risk of calcium stone formation [177]. Metabolic acidosis has been reported in up to 10% of ileal conduit diversions [178], whereas in continent urinary diversions it may be as high as 50%, due to longer periods of contact between urine and intestinal mucosa [179].
Medical Management
Increased fluid intake, correction of metabolic abnormalities, and prophylaxis against recurrent infections are crucial in avoiding recurrent stone formation. Prevention of uric acid and calcium stone formation as a result of acidosis may be achieved by alkali supplementation (e.g. potassium citrate). CaOx stone formation as a result of enteric hyperoxaluria may be treated by measures to control fat‐malabsorption and by increasing calcium intake at meals, thereby reducing intestinal hyperabsorption of oxalate.
Surgical Management
In principle, stones in patients with urinary diversions can be managed similarly to other stone patients.
Renal and Ureteric Stones SWL may be used for smaller renal and ureteric stones in cases without strictures distally to the stone. Retrograde ureteric access is often challenging due to difficulty in locating the neo‐ureteric orifices. In general, ureteric access is more easily achieved in ileal conduits than in reservoirs owing to the lack of an afferent limb [169].
In case of anastomosis‐stricture, an antegrade or a combined antegrade‐retrograde approach is usually the best choice. Percutaneous access is best obtained ultrasonographically because retrograde opacification of the collecting system with contrast medium is often impossible. For access to ureteric stones, flexible scopes are usually necessary, and the use of a ureteric access sheath may ease the procedure dramatically.
Stones in Neobladders and Reservoirs Stones in orthotopic diversions may be approached transurethrally. For small stones in reservoirs a trans‐stomal approach using small‐calibre rigid or flexible endoscopes may be an option. For larger stones, a trans‐stomal access is usually not advisable because these will require significant manipulation and usage of larger scopes, which may disturb the continence mechanism. In such cases, a percutaneous access guided by ultrasound or cystoscopic guidance usually is preferred [169].
Stone‐Free Rate and Residual Stones The main goal of stone treatment is to render the patient stone free, and stone‐free rate (SFR) is the key parameter for success outcome. Results expressed as SFR in the literature varies a great deal, partly due to variability in follow‐up imaging and in definitions.
The term ‘stone free’ ideally is defined as 100% free of any stone material, but there is no consensus or standardised definition. In published series the definition of stone free varies or is often not accounted for [180], and SFR often include cases with minor residual fragments (RF).
The term ‘clinically insignificant residual fragments’ (CIRF) was introduced in 1986 with SWL and is most often defined as small asymptomatic nonobstructive, noninfectious residual fragments. The defined maximum size of fragments however varies from ≤3 to ≤5 mm [181–183].
CIRF may also be present after PCNL and retrograde intra renal lithotripsy.
Methods to increase fragment clearance immediately following stone treatment include medical expulsive therapy (MET) and inversion therapy [25, 26].
Imaging in the follow‐up after stone treatment may include ultrasound plain X‐ray (KUB), tomography or NCCT. The detection of RF depends on the imaging modality applied. The sensitivity of NCCT is close to 100% [184]. The sensitivity of plain X‐ray, tomography, and ultrasound is 62.9, 74.3, and 48.6%, respectively, in radio‐opaque stones. For weak radio‐opaque or radiolucent stones the sensitivity was 11.1, 22.1, and 22.2%, respectively [184]. Comparing ultrasound with NCCT in detecting renal calculi, a sensitivity of 26% was found for stones 3–7 mm, and 71% for stones >7 mm [185].
The measured size of residuals is also dependent on imaging modality. CT accurately estimates transverse size but overestimates the craniocaudal size; KUB overestimates size; tomography underestimates size; and ultrasound tends to overestimate size [185–189].
Several studies have investigated the fate of RF, and it has become evident that the term CIRF is a misnomer because RF often become significant with time. RF may dislodge resulting in pain or ureteral obstruction or may serve as a nidus for new stone formation or persistent infection [93, 94, 190, 191]. Ranging from 20 to 60% of patients with RF required treatment within five years [92–94, 190, 191]. The risk is much higher in infection stones [192].
Adequate preventive measures depending on stone analysis and metabolic evaluation should be made to decrease the risk of regrowth [95, 96, 193, 194]. Long‐term follow‐up of patients with RF must be personalised depending on individual risk factors. It is recommended that patients with RF should be followed up regularly [25, 26].
Pregnancy
The presence of urolithiasis during pregnancy is rare with an incidence ranging from 1 in 1500 to 1 in 2000 [195]. Compared to the lifetime risk of developing urolithiasis in the non‐pregnant population (1–10%), the risks during pregnancy are much lower, ranging between 0.03 and 0.53% [195]. The diagnosis of urolithiasis in pregnancy is usually made after the first trimester when the disease becomes symptomatic.
Urolithiasis is the second most common cause of abdominal pain in the pregnant patient after UTIs, but it is the most common cause of nonobstetric reason for hospital admission [195].
During pregnancy, the body undergoes a series of anatomical and physiological changes that may be associated with an increased likelihood of stone formation. The ureters dilate as early as the first trimester and remain dilated throughout pregnancy. This allows the migration of any renal stones down into the ureters, leading to obstruction or pain. As well as migration, the dilated ureters can lead to urinary stasis, thereby facilitating the aggregation of urinary crystals.
There is also an increase in GFR during pregnancy by 30–50%. This increased GFR results in more sodium, calcium, and uric acid filtered by the kidneys. This, with the anatomical changes further increases the likelihood or urinary crystal formation. However, this increase in GFR does also increase the urinary excretion of citrate, glycoproteins, and magnesium, which have been documented to inhibit stone formation both in vivo and in vitro.
The physiological hydronephrosis caused by the enlarged uterus during pregnancy causes diagnosis of intramural obstruction of the ureter difficult. With distal ureteric stones, where the obstruction may be below the pelvic brim and knowing that pregnancy‐related hydronephrosis does not tend to go this low, the diagnosis is easier than with proximal or mi‐ureteric stones.
The presence of stones that reside in the urinary tract can lead to renal colic, infection, and obstruction, which pose significant risks to both mother and child.
Diagnostic modalities remain a dilemma due to the fear of exposing the foetus to ionising radiation that can cause foetal malformations, gene damage‐causing mutations, or even cancer in later life. The foetus is most at risk during the early development of organs (organogenesis) during the first trimester. However, radiation exposure <50 mGy is not associated with an increased risk to the foetus and considered negligible [196, 197]. Nonetheless, most urologists and radiologists will obtain an ultrasound. In rare instances, plain file, NCCT, or magnetic resonance urogram can help with the diagnosis.
When managing a pregnant patient with urolithiasis, conservative management is favoured where possible. This is because 70–80% of stones have been shown to pass spontaneously [195]. The mainstay of conservative management is for rehydration, anti‐emetics, analgesia, and antibiotics if an infection is suspected.
In the remaining 20–30%, surgical intervention is required. The indications for surgical intervention are for those that do not improve with conservative measures, such as infected hydronephrosis with declining renal function or uro‐epsis [195].
Temporisation methods:
- Ureteric stent insertion
- Percutaneous nephrostomy
Either under local anaesthetic, sedation, or a short general anaesthetic. However, this will need to be changed regularly, at least every six to eight weeks, due to the high risk of encrustation. However, both options are poorly tolerated.
Definitive treatment:
- URS
With the advancements in technology and endourological techniques, URS has become safer with ever‐improving results with stone‐free rates as high as 86% [195]. However, the risk might still be considered high with a 8–16% developing procedure related complications, as well as obstetric complications, including premature uterine contractions or even premature delivery [195].
PCNL is best avoided, and SWL is a contraindication.

Full access? Get Clinical Tree


