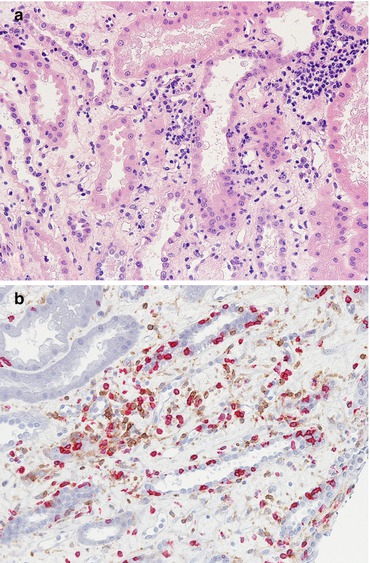
Fig. 21.1
A panoramic view of the donor biopsy stained with H&E (a), PAS (b) and trichrome stain (c) showing the ischaemic subcapsular area with inflammatory infiltrate, moderate fibrosis and obsolescent and sclerotic glomeruli. The punch biopsy can sometimes overestimate the real state of the kidney if the biopsy falls in such areas. Magnification 2×
The pathological report of the donor biopsy includes:
Glomerular disease
The percentage of sclerotic glomeruli. Glomerulosclerosis alone is not an independent predictor of graft function [2] even though glomerulosclerosis >10 % correlates with a major rate of delayed graft function (DGF), primary nonfunction and graft loss [3]. The degree of glomerulosclerosis can be a predictor of serum creatinine value at 12 months [4].
Any thrombotic microangiopathies (TMA) (Fig. 21.2). Glomerular fibrinoid thrombi can be dissolved by the graft recipient’s fibrinolytic system without any anticoagulant therapy. In one series, some recipients experienced a primary nonfunction, while others had an initial DGF without any impairment of graft function at 2 years [2, 5, 6].
Any glomerular disease, i.e. lupus nephritis or diabetic glomerulonephropathies (Fig. 21.3). These features do not enter into the Karpinski score. Some studies suggest that diabetic kidneys can be used safely and that early diabetic lesions can regress with transplantation. However, no guidelines are currently available on the use of diabetic kidneys [7–9].
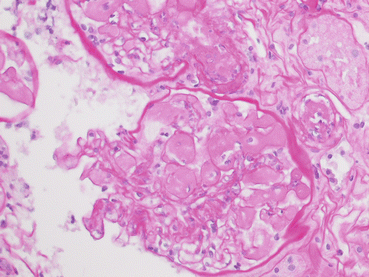
Fig. 21.2
At high magnification (PAS, 40×) the capillary lumen of the glomerulus is filled with a pale amorphous material extending into the vascular pole, occluding the lumen
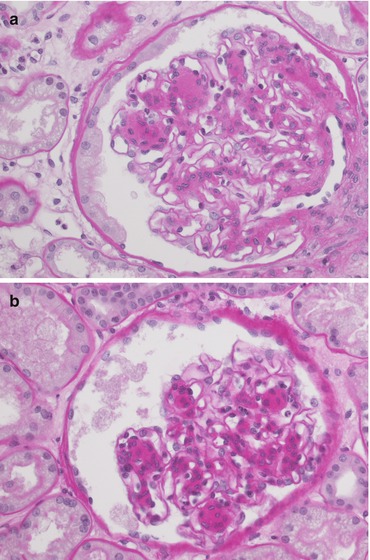
Fig. 21.3
The pictures (a, b) highlight the mesangial expansion with a nodular appearance. This feature does not enter into the Karpinski score. The biopsy in other fields showed just a mild interstitial and arterial intimal fibrosis without remarkable tubular atrophy. Magnification 40×, PAS
Vascular disease
Tubulointerstitial disease
At our centre all these parameters are evaluated according to the Karpinski score system [12].
Glomerular score | |
Score 0 | No globally sclerotic glomeruli |
Score1 | <20 % globally sclerotic glomeruli |
Score 2 | 20–50 % globally sclerotic glomeruli |
Score 3 | >50 % globally sclerotic glomeruli |
Tubular score | |
Score 0 | Absent |
Score 1 | <20 % of tubules affected |
Score 2 | 20–50 % of tubules affected |
Score 3 | >50 % of tubules affected |
Interstitial score | |
Score 0 | No evidence of fibrosis |
Score 1 | <20 % of cortical parenchyma replaced by fibrotic tissue |
Score 2 | 20–50 % of cortical parenchyma replaced by fibrotic tissue |
Score 3 | >50 % of cortical parenchyma replaced by fibrotic tissue |
Vascular score(arterial fibrosis and arteriolar hyalinosis are evaluated, but the most severe lesion determines the final score) | |
Score 0 | Absent |
Score 1 | Increase in wall thickness that is less than the diameter of the lumen |
Score 2 | Increase in wall thickness that is equal to the diameter of the lumen |
Score 3 | Increase in wall thickness that exceeds the diameter of the lumen |
21.1.1 Other Predictive Factors
Prediction of ischaemic injury effects
Neutrophils in glomerular capillaries
Endothelial loss of capillaries (correlated to a transiently impaired function)
Tubular degeneration: necrosis or apoptosis of tubular cells, regenerative features with enlargement of tubular cell nuclei and simplification and loss of the brush borders of distal tubular cells
Tubular CAST (precipitation of proteinaceous amorphous/detritic material in renal tubules)
Microvascular thrombi in capillaries and small arteries without granulocytes
Prediction of acute rejection
Neutrophils, macrophages and platelets in capillaries (glomerular and peritubular).
C4d deposition in peritubular capillaries may be predictive of acute antibody-mediated rejection (AMR).
Prediction of DGF
Thrombotic angiopathy in glomerular capillaries
21.2 Surgical Complications
21.2.1 Ureteral Obstruction/Leak/Reflux
The presentation is with oliguria, haematuria and elevated creatinine. At histology only non-specific alterations can be seen: interstitial inflammation, oedema and tubular injury.
21.2.2 Lymphocele
Lymphocele consists in a collection of lymphatic fluid in the perinephric space. At histological examination the renal biopsy shows changes related to obstruction, with interstitial oedema, dilatation of the collecting ducts and mild inflammation.
21.2.3 Arterial/Venous Thrombosis
The clinical presentation is of macrohaematuria and acute renal failure. At histology, microthrombi and loss of the endothelium can be seen together with haemorrhage of the cortex and mild neutrophils in capillaries. The most important differential diagnosis is with acute cellular rejection (ACR) and hyperacute or AMR. Imaging findings (US and angiography) and immunohistochemistry for C4d lead to the correct diagnosis.
21.2.4 Arterial Stenosis
The clinical presentation is of DGF, hypertension and renal dysfunction. The histological picture may disclose cholesterol emboli (if the stenosis is due to an atheromatous plaque). Non-specific tubular atrophy with fibrosis or acute tubular injury (if the stenosis is intermittent) is evident.
21.3 Rejection
21.3.1 Hyperacute Rejection
Nowadays the incidence of hyperacute rejection is very low due to improved pre-transplant testing for antibody against donor major antigens. The presentation is very rapid: from a few minutes (at the time of graft reperfusion) to a few days after transplantation, depending on the recipient’s titres of specific antibody against the donor antigens. Anuria, primary graft nonfunction, fever, lack of perfusion by imaging studies, thrombocytopenia and increased circulating fibrin split characterize the clinical onset. At gross examination the graft is cyanotic, flaccid, haemorrhagic and oedematous with necrotic areas. Thrombosis of the renal artery can be found.
21.3.1.1 Histological Examination
Early features (1–12 h):
1.
Platelet and neutrophil margination in glomerular and peritubular capillaries
2.
Vascular congestion with scattered thrombi in glomerular capillaries and small arteries
Late features (12–24 h) include:
1.
Interstitial oedema and haemorrhage
2.
Widespread thrombotic microangiopathy
3.
Fibrinoid arterial necrosis
4.
Parenchymal necrosis
21.3.1.2 Ancillary Techniques
1.
C4d peritubular capillaries and glomerular IHC staining.
2.
Negative staining does not exclude a hyperacute rejection since C4d-negative cases are possible (related to decreased perfusion or to insufficient time to produce the C4d molecule, a product of the activation of the classical complement pathway).
21.3.1.3 Differential Diagnoses
1.
Renal artery or vein thrombosis due to technical problems or hypercoagulable state (in these cases the thrombi are limited to the large vessels, and C4d staining is negative).
2.
Perfusion nephropathy with loss of the endothelium. In this case congestion and thrombotic microangiopathy can be found without C4d staining.
3.
Donor thrombotic microangiopathy.
21.3.2 Acute Humoral Rejection or Acute Antibody-Mediated Rejection
About 5–7 % of transplanted patients experienced an episode of antibody-mediated rejection (AMR) and a component of humoral rejection can be found in at least about 24 % of acute rejection cases. Presentation occurs from a few days to the first weeks after transplant, with anuria and acute renal failure. Circulating anti-donor-specific antibodies can be detected in about 90 % of recipients. At gross examination the graft is swollen, haemorrhagic and oedematous.
21.3.2.1 Histological Examination
Glomeruli
Tubules
Acute tubular injury represented by a simplification of tubular cells with loss of the brush border, ischaemic necrosis with loss of nuclei, thinning of the tubular cell cytoplasm and naked basal membranes
Tubulitis with neutrophil infiltrate
Peritubular capillaries: dilatation and congestion of capillaries filled with neutrophils and occasional microthrombi
Interstitium: oedema, occasionally haemorrhagic areas, inflammatory infiltrate predominantly with neutrophils
Vessels
Necrosis of the media with fragmentation of the lamina elastica and fibrinoid necrosis
Inflammatory cells within the vessel wall
Activation of capillary and artery endothelial cells showing plump cytoplasm and nuclear enlargement
21.3.2.2 Ancillary Techniques
IHC positivity for C4d along the peritubular and glomerular capillaries (Fig. 21.4). Early biopsies may not disclose C4d deposition, but this does not rule out a diagnosis of AMR since this may become positive in repeated biopsies taken after 1–3 days. Conversely, some studies have demonstrated that positive immunostaining for C4d in protocol biopsies appears before the clinical manifestation of an AMR episode [17].
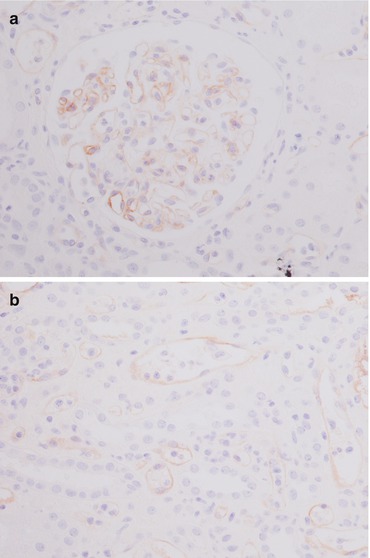
Fig. 21.4
Immunohistochemical stain for C4d shows a diffuse and continuous linear positivity in glomerular (a) and along peritubular capillaries (b). Magnification 20×
IHC staining for CD68 does not improve the sensitivity of identifying glomerulitis when the Banff 2013 definition is applied.
Wide and diffuse immunopositivity for C4d at immunofluorescence (IF), which is more sensitive than IHC.
21.3.2.3 Diagnostic Criteria (Banff Classification)
The definition of AMR has now been revised [15]: histological evidence of acute tissue injury (glomerulitis and/or peritubular capillary infiltration and/or acute thrombotic microangiopathy and/or tubular necrosis) (Fig. 21.5) must be present together with evidence of a recent endothelial/antibody reaction (positivity for C4d IHC and/or microvascular inflammation highlighted by glomerulitis or peritubular capillaritis) and serological evidence of anti-donor-specific antibodies.
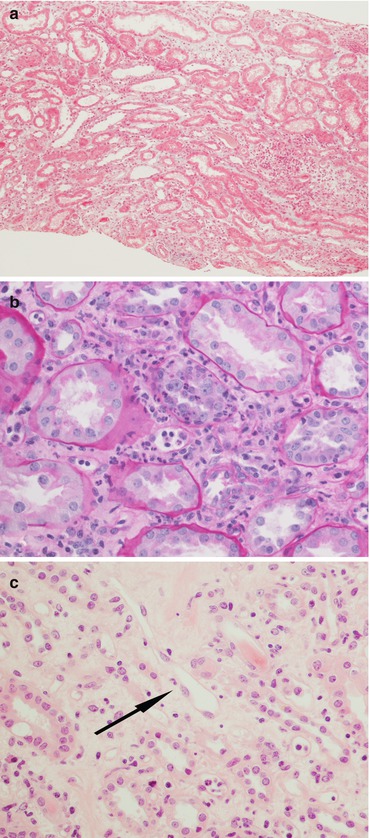
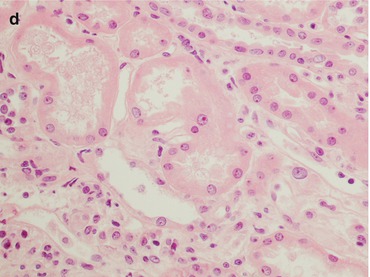


Fig. 21.5
In AMR there is a diffuse interstitial oedema highlighted by the pale light green trichrome stain (a magnification 4×), tubulitis with neutrophil infiltrate (b PAS, magnification 20×), activation of the endothelial cells (arrow, c H&E, magnification 40×) and acute tubular necrosis (d H&E, magnification 40×)
The histological pattern can be graded from grade 1 with acute tubular damage and minimal inflammatory infiltrate to grade 2 with peritubular capillaritis or glomerulitis with occasional microthrombi or grade 3 with fibrinoid arterial necrosis. The presence of only one or two of these criteria leads the pathologist to a diagnosis of “suspect” AMR (Fig. 21.6).
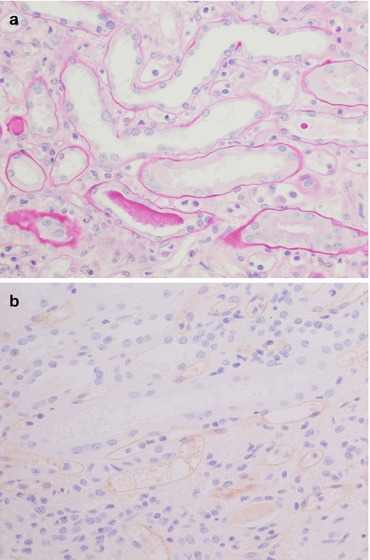

Fig. 21.6
This is a biopsy 5 days after kidney transplantation in a patient with oliguria. The histological pictures (a PAS, magnification 40×) demonstrated only an acute tubular necrosis of the parenchyma with a mild peritubular capillaritis without any interstitial inflammatory infiltrate or tubulitis. The main differential diagnosis is with a tubular cell necrosis with a delayed graft function. The C4d stain (b magnification 40×) reveals a diffuse deposition of the molecule along the peritubular capillaries: the patient was given a steroid bolus before the biopsy because of the clinical suspicion of AMR
Biopsies with a C4d-positive immunostaining without any inflammation can be considered a kind of “accommodation”.
21.3.2.4 Differential Diagnoses
Acute cellular rejection: generally the infiltrate is composed predominantly of T lymphocytes. Fibrinoid necrosis of renal small arteries, glomerulitis, microvascular thrombosis and areas of infarction are less prominent [18].
Chronic humoral rejection: the clinical setting shows a slow decline.
Accommodation: C4d deposition in the absence of an inflammatory reaction.
Acute tubular injury/acute tubular necrosis: simplification of the renal tubules with loss of nuclei, thinning of tubular cell cytoplasm and naked basal membranes in the absence of an inflammatory reaction. C4d is negative.
Acute pyelonephritis: dirty casts in the tubules (composed of neutrophils and necrotic material) with a diffuse infiltrate of neutrophils in the parenchyma. A positive urine culture and C4d negativity favour the diagnosis.
Thrombotic microangiopathies: due to drug toxicity or recurrent disease. There is no C4d immunoreactivity.
21.3.3 Acute Cellular Rejection or T-Cell-Mediated Rejection
Acute cellular rejection (ACR) involves 5–10 % of kidney recipients in the first year posttransplant, generally in the first few weeks. The frequency tends to decline after the first 6 months, but ACR can arise at any time in the recipient. In acute renal failure increased serum creatinine concentration, decline in urine output and oliguria, weight gain, fever and malaise define the clinical picture. Circulating anti-donor-specific antibodies can be detected in about 90 % of recipients. At US the graft becomes enlarged, oedematous and tender. At gross examination the graft is swollen, pale, haemorrhagic with congestion of the medulla, and oedematous. Foci of infarction may be evident on the cortex.
21.3.3.1 Histological Examination
Glomeruli
Only occasional mild inflammatory infiltrate of mononuclear cells (generally in only 10 % of cases) in glomeruli with mild reactive swelling in endothelial cells (Fig. 21.7a).
In <5 % of cases a severe mononuclear cell glomerular infiltrate (acute allograft glomerulopathy) can be found, giving the appearance of endocapillary hypercellularity. Endothelial injury with areas of mesangiolysis is associated. This picture is often seen in the setting of moderate ACR with endoarteritis, but it can also represent the only histological feature of rejection.
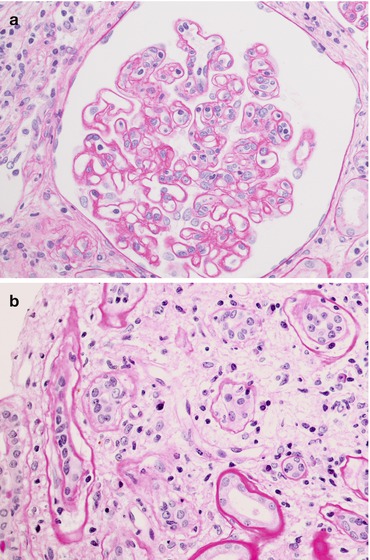
Fig. 21.7
The picture on the left (a PAS, magnification 40×) shows a mild inflammatory infiltrate of mononuclear cells in the glomerulus, while the picture on the right (b PAS, magnification 40×) shows marked tubulitis: note the lymphocytes between the tubular epithelium and the basement membrane and the moderate interstitial oedema
Tubules
Tubulitis with T-cell lymphocytes (CD8+) (Fig. 21.7b)
Tubular cell injury, occasionally with a granulomatous reaction
Interstitium
Oedema, occasionally with haemorrhagic areas.
Chronic inflammatory infiltrate (involving at least 25 % of the cortex) with a predominance of T-cell lymphocytes and macrophages (Fig. 21.8). Macrophages/monocytes can be the major cell type, notably in the setting of T-cell-depleting drugs (such as CAMPATH 1).
A plasma cell-rich subset and a CD20+ B-cell-rich subset AR have a worse prognosis.