Chapter 3.19
Irritable bowel syndrome dietary management
Heidi Staudacher1 and Gareth Parkes2
1 King’s College London and Guy’s and St Thomas’ NHS Foundation Trust, London, UK
2 Barts Health NHS Trust, London, UK
3.19.1 Dietary effects of disease or its management
Up to 90% of patients with irritable bowel syndrome (IBS) identify food as having an important role in the generation of symptoms [1–3] and many patients report a preference for dietary management rather than reliance on medical therapy. As a result, self-management is frequently attempted, which almost always involves exclusion of food(s) in the absence of a rationale. Therefore, although IBS does not inherently affect nutritional status, attempts to self-manage symptoms via dietary means may do so.
Up to 50% of patients alter their diet to improve symptoms [4]. Many foods have been reported to trigger symptoms in IBS patients including dairy, grains, fruit, vegetables and egg [1,5]. Commonly reported trigger foods are often rich in important nutrients, so it is unsurprising that nutritional inadequacies become apparent on assessment of dietary intake. Low intake of B vitamins, iron and/or calcium [6–8] has been reported, although not all studies are in agreement [9]. Heterogeneity of studies and the lack of research limit definitive conclusions. However, it is generally well accepted that dietary intake may be at risk, leading to nutritional deficiency.
Guidelines for the management of IBS state that diet and lifestyle should be first-line considerations [10,11]. In most cases, a dietitian will investigate clinical history, anthropometry, symptom profile, dietary pattern, nutritional intake and previously implemented dietary measures prior to providing dietary advice. Determining the level of motivation for dietary modification is important. A diet and symptom diary may help identify poor eating patterns and determine whether food is implicated in symptom provocation, although this is not always a valid measure of identifying problem foods. Various dietary intervention strategies are available for IBS patients and a clinical algorithm for dietary management has been developed (Figure 3.19.1) [10].
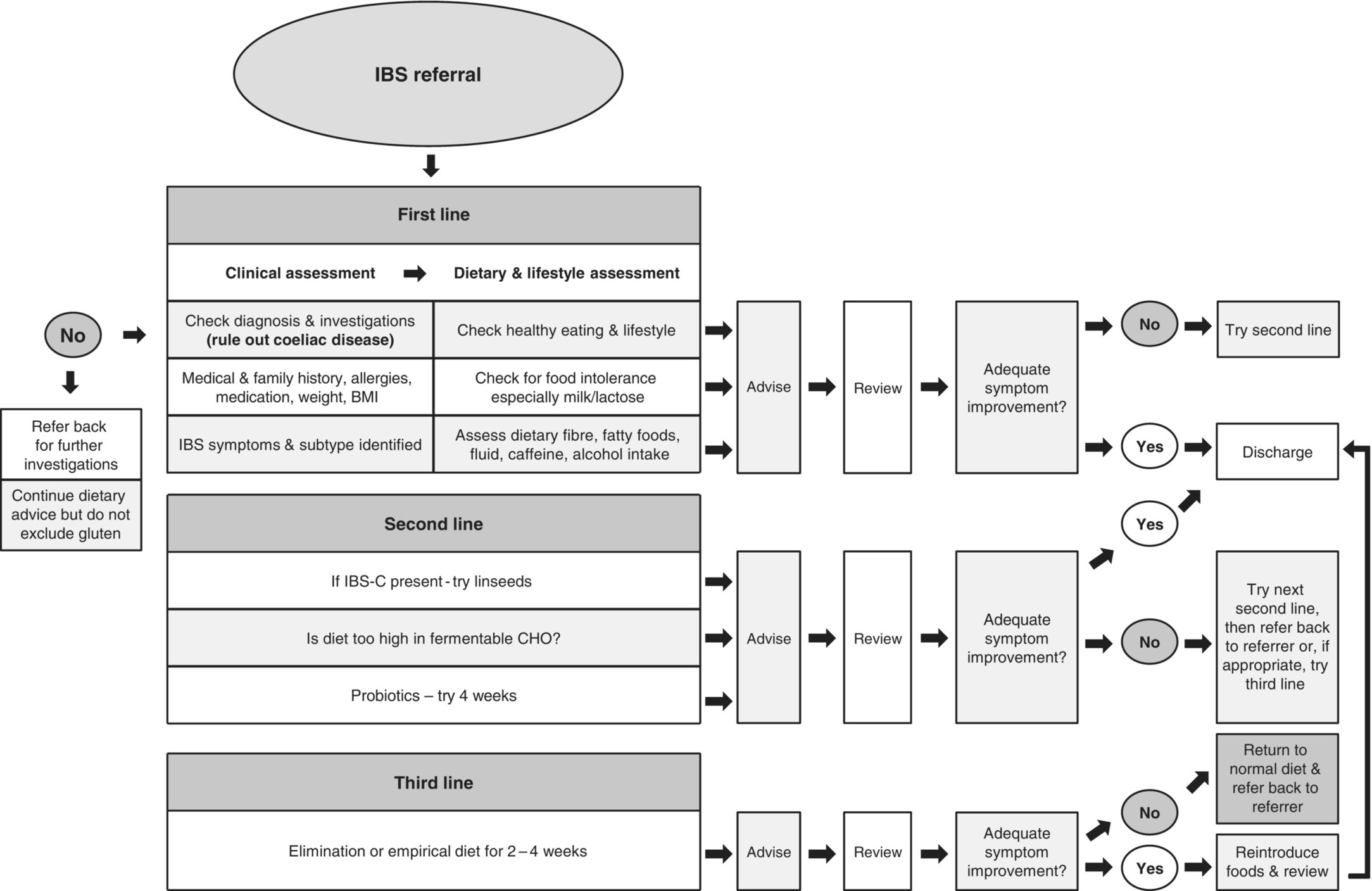
Figure 3.19.1 Algorithm for dietary management of IBS. BMI, Body Mass Index; CHO, carbohydrate; IBS-C, constipation-dominant irritable bowel syndrome.
Possible underlying pathophysiological mechanisms of IBS include visceral hypersensitivity [12], altered colonic motility [13], altered fermentation [14] or disturbed gas handling [15] and therefore it is not surprising that symptoms are generally induced postprandially [16]. Furthermore, food-related symptoms are more frequent in those with anxiety [16]. Assessment of eating behaviour (e.g. frequency, pattern, the eating environment) is important as symptoms may lead to reduced frequency of meals and greater likelihood of overeating which might exacerbate symptoms. Advice regarding eating at regular intervals and appropriate eating behaviours forms the basis of first-line dietary guidelines [10]. Sitting down to eat and eating slowly, chewing food thoroughly and avoiding eating late at night may be very useful. Explanation of the underlying pathophysiology of IBS may reassure patients and prevent unnecessary restriction of foods when poor eating behaviour is suspected as a primary trigger of symptom generation.
3.19.2 Dietary interventions
Lactose
Lactose malabsorption is present in up to 50% of patients with IBS, depending on ethnicity [17]. Lactose hydrogen breath testing can be useful to diagnose lactose malabsorption but may not be available. To assess lactose intolerance, a lactose food challenge using at least 12 g lactose (e.g. 125 mL milk) and a food and symptom diary to identify symptom exacerbation may be useful. Other dietary and lifestyle factors are also often implicated, and therefore exclusion of high-lactose foods may not lead to symptom resolution.
Non-starch polysaccharides
Historically, non-starch polysaccharides (NSP) have been a target for dietary intervention in IBS, particularly in those with constipation or with variable stool output (i.e. alternating constipation and diarrhoea). However, a recent Cochrane review suggested that bulking agents, whether insoluble (e.g. wheat bran) or soluble fibre (e.g. psyllium, ispaghula), are not effective in improving IBS symptoms [18] Indeed, wheat bran may worsen symptoms and the addition of insoluble fibre for patients with IBS is now discouraged [11].
Studies using soluble fibre supplementation indicate that ispaghula husk may be useful in patients with constipation [19] but more recent reviews for its use in IBS present the overall evidence as weak. Studies are often hindered by either the lack of a control group or high placebo response rates. Interpretation of results is difficult due to study heterogeneity. For example, studies differ with respect to patient symptom profiles, the source of supplemental NSP, duration of intervention and outcome measures. Poor study design and high drop-out rates are also common. Furthermore, clinically, supplementation with soluble fibre may induce bloating and discomfort probably due to its fermentation in the large intestine, although individual symptom response appears to vary markedly.
Constipation-predominant IBS is notoriously difficult to manage via dietary means. The evidence for NSP supplementation is weak but should not be ignored. Supplementation with 50 g/day linseed (flaxseed) increases the number of bowel movements in a healthy population by 30% [20] and 24 g/day linseeds improves constipation more than psyllium over 3–6 months in constipation-predominant IBS patients, although laxative use was not monitored in this study [21]. Recently, a small open-label randomised pilot study showed that intake of ground or whole linseed for 4 weeks was associated with improvement in IBS symptom scores compared to no linseeds in a group of mixed IBS subtypes [22]. This was not statistically significant in the intention-to-treat analysis and further work is needed to verify these results and investigate the effect of linseeds on stool output in constipation-predominant IBS.
Conversely, NSP restriction should reduce stool bulk and frequency and is used to manage diarrhoea-predominant IBS with some good effect. Research on NSP restriction in enterally fed hospital inpatients with diarrhoea and a 2-week NSP-free liquid diet showed an improvement in overall symptom scores in IBS [23]; however, this is impractical to maintain in the long term. Formal investigation into its efficacy, the level of restriction required or nutritional consequences of its implementation has not been performed.
Caffeine
Caffeine is a stimulant of colonic motility in healthy individuals [24] and it is likely that sensitivity and symptom response are exaggerated in those with IBS. There are no randomised controlled trials investigating the effect of restriction of caffeine on symptoms in IBS. However, many patients report symptoms with caffeine consumption [1,5,16] and often limit or avoid it. Exclusion diet studies demonstrate that reintroduction of caffeinated drinks induces symptoms in approximately 30% of patients with IBS [5,25]. Dietary guidelines suggest a trial of caffeine restriction and encourage fluids from non-caffeinated sources.
Alcohol
Alcohol is a perceived symptom trigger in up to 33% of patients with IBS [5,16]. A large cross-sectional study in the community did not find an association with alcohol intake and the incidence of IBS, although an increased likelihood of abdominal pain with moderate intakes of alcohol was found [26]. This does not demonstrate causality and randomised controlled studies on the effects of alcohol in IBS have not been performed. Large individual variation in tolerance is apparent not only to volume but types of alcoholic drinks. It would seem prudent that patients abide within healthy limits for alcohol consumption and specific advice should be given on a case-by-case basis.
Fat
Dietary fat is often a trigger for GI symptoms in patients with IBS, particularly after meals that contain large amounts of cream, oil, butter or foods that are battered or crumbed [1,3,5]. Fat is a stimulant of colonic motility and impairs gas transit in IBS [27]. Furthermore, duodenal lipid infusion results in lower colonic pressure thresholds in IBS patients versus controls, leading to earlier symptoms [28]. However randomised controlled dietary studies are required to confirm that restriction is indeed effective.
Resistant starch
Most dietary starch is completely digested in the small intestine but a fraction, termed ‘resistant starch’ (RS), survives digestion and contributes to NSP intake [29]. It has similar effects to other NSP in the GI tract, including increased stool bulk [30,31], osmotic pull and fermentative effects leading to increased short-chain fatty acid production [30]. There are a number of classifications, including type 1 which is present in foods where the starch is physically inaccessible, e.g. in seeds. Type 2 RS is found in raw starch granules, e.g. raw potato. Type 3 is formed when amylase and amylopectin are retrograded after heating and subsequent cooling and is present in foods such as bread, cooked and cooled potato and cornflakes. More recently, chemically modified starches have been included in the definition and are classified as type 4 RS and are present in some high-fibre fluids and breads.
Most studies have focused on the physiological effects of RS in healthy individuals and it has been demonstrated that high RS intakes of up to 60 g/day can lead to GI symptoms, including bloating, increased stool frequency and loose stools [32]. This may partly be due to its fermentation in the large intestine. However, the fermentation rate is relatively slow [33] and as a polymerised structure with a high molecular weight, it is usually better tolerated than other indigestible carbohydrates. The effect of RS in patients with IBS has not been investigated. However, anecdotal evidence suggests it might induce symptoms in some patients. Patients reporting symptoms after consumption of foods high in RS (e.g. part-baked breads, pizza, pasta and convenience foods) sometimes respond to avoidance of these foods. However, whether it is the RS or another component that triggers symptoms is unknown and research is warranted to further delineate its role in inducing symptoms.
Fermentable carbohydrates
There is growing interest in the effects of restricting short-chain fermentable carbohydrates, collectively termed FODMAPs (fermentable, oligo-, di- and monosaccharides and polyols), for the management of IBS. Chemical structure, physiological effects in the GI tract and sources of these carbohydrates are described in Chapter 2.2. Many studies have investigated the effect of individual fermentable carbohydrates on GI symptoms in patients with IBS. For example, fructose, sorbitol or fructans, either individually or in combination, are associated with exacerbation of symptoms [34,35,36] and restriction is associated with improvement in functional GI symptoms [37,38]. More recently, research has focused on the effect of avoiding fermentable carbohydrates collectively (i.e. low FODMAP diet) which is effective in improving overall symptoms in up to 70% of patients in randomised controlled trials and up to 94% in patients in uncontrolled work [39]. Gas-related symptoms such as bloating and wind, and urgency and stool frequency appear to be particularly responsive to this type of dietary restriction.
A diet low in FODMAPs involves the avoidance of fructans, galacto-oligosaccharides, polyols, fructose in excess of glucose, and lactose. Poor absorption of fructose and lactose only occurs in a proportion of patients and restriction should occur based on the results of lactose and fructose hydrogen breath testing or clinical suspicion. Dietitian-led education regarding FODMAP restriction should occur with the intervention lasting at least 4 weeks [6]. Although the diet requires substantial effort, recent work has shown that it is no more difficult to follow or understand compared to standard IBS advice [40].
After a period of FODMAP restriction, patients follow a staged reintroduction process, systematically reintroducing foods high in one short-chain fermentable carbohydrate. This helps to determine dose tolerance and ultimately directs long-term dietary habits whilst promoting increased nutritional variety.
Due to the complex nature of the low FODMAP diet, and that foods frequently consumed require restriction (i.e. some breads and cereals, fruits and vegetables, dairy products), it has been suggested that this diet may not be nutritionally adequate, which is always a risk with elimination diets. Indeed, a lower calcium intake was found in patients with IBS following a low FODMAP diet compared to a habitual diet [6]. This is a concern as IBS may be associated with a higher risk of osteoporosis [41]. Emphasis should be placed on inclusion of appropriate portions of high calcium, low lactose foods. Specific instruction on suitable brands of low lactose dairy alternatives.
A low FODMAP diet also affects the GI microbiota and recent work has shown that luminal bifidobacteria are markedly lower after a 4-week low FODMAP diet in patients with IBS [6]. This is probably due to the restriction of prebiotic fructo- and galacto-oligosaccharides. Whether this effect persists long term or affects long-term health is not known and requires further investigation.
Finally, research on the efficacy of FODMAP restriction has thus far been limited to a dietitian-led approach. Compliance, which is influenced by patient motivation but also careful advice from an experienced dietitian who has access to up-to-date lists of suitable and unsuitable foods, is vital to its success. The success of a simpler modified approach when complete restriction is not justified, led either by a dietitian or other health practitioner, is yet to be determined.
Gluten
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






