CHAPTER 118 Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is an important disease entity because of its high prevalence, substantial morbidity, and enormous costs.1–3 In the United States, approximately 12% of patients seen by primary care physicians have IBS, but it is likely that this frequency is an underestimate.4–6 In gastrointestinal practices, more than one third of patients have functional gastrointestinal disorders, IBS being the most common diagnosis.7 Because a substantial proportion of gastroenterology practice comprises patients with IBS or other functional gastrointestinal disorders, it is essential that clinicians develop expertise in their diagnosis and treatment. The diagnosis of IBS rests on making a positive clinical diagnosis from the history; that tests often are not needed represents an important conceptual advance.8 There is increasing evidence that at least a subset of IBS has an organic basis in the gastrointestinal tract.9 Nonetheless, only symptom-directed therapy rather than disease-modifying treatments are available; the evidence base for current therapy has strengthened considerably with the publication of well-performed meta-analyses. In this chapter, current knowledge of the epidemiology and pathophysiology of IBS is reviewed to provide a rational basis for its diagnosis and therapy.
DEFINITIONS
IBS is characterized by the presence of abdominal discomfort or pain associated with disturbed defecation.3 Bloating or visible abdominal distention often is present in patients with IBS but are not considered essential symptoms for diagnosis.3 Furthermore, individual symptoms are neither sensitive nor specific enough on their own to diagnose IBS.10
In a classic study from the United Kingdom, Manning and associates first reported that six symptoms were more common in patients in whom IBS was subsequently documented, although only four were statistically significant in the initial report (Table 118-1).11 Later studies showed that these symptoms were specific, but not sensitive, for identifying IBS and were of greater diagnostic value in women.10,12 The Kruis scoring system is based on the presence and duration of symptoms, negative physical examination findings, and normal simple laboratory tests, and it has modest diagnostic utility (see Table 118-1).13
Table 118-1 Manning, Kruis, and Rome III Criteria for Irritable Bowel Syndrome
| Manning Criteria* |
| Kruis Criteria |
| Patient’s History |
| Physician’s Assessment† |
| Rome III Criteria‡ |
Recurrent abdominal pain or discomfort§ at least three days/month in the last three months associated with two or more of the following: |
Kruis criteria adapted from Kruis W, Thieme C, Weinzierl M, et al. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology 1984; 87:1-7.
Rome III criteria from Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. In: Drossman DA, editor. Rome III: The Functional Gastrointestinal Disorders. 3rd edition. McLean, VA: Dagnon Associates. 2006, pg 491. Used with permission from the Rome Foundation.
* Diagnostic cut-off: three or more of the six symptoms listed.
† If any abnormal physical findings or any of the laboratory parameters assessed by the physician are present, IBS is excluded.
‡ Criteria fulfilled for the previous three months, with symptom onset at least six months before diagnosis.
§ “Discomfort” means an uncomfortable sensation not described as pain. In pathophysiology research and clinical trials, a pain or discomfort frequency of at least two days a week during screening evaluation is recommended for subject eligibility.
Manning criteria adapted from Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. BMJ 1978; 2:653-4.
In an effort to build on the diagnostic utility of the Manning and Kruis criteria, the Rome (I, II, and III) criteria were created following a formal consensus process to provide a standard for clinical research (see Table 118-1).3 The Rome criteria are useful in clinical practice and can be used to make a positive clinical diagnosis.1,3 The sensitivity and specificity of the Rome I criteria have been reported to be 71% and 85%, respectively,14 and although adequate validation data for Rome III are lacking, the main criteria for Rome II and III are very similar.10 Comparisons of the criteria have shown that Rome I and II criteria are specific and identify similar patient populations, although, compared with Rome I criteria, the Rome II criteria appear to identify fewer cases in some studies.15–17 The Manning criteria identify additional patients with IBS-like symptoms who do not fulfill any of the Rome criteria but arguably also should be classified as having true IBS.15–17
CLINICAL FEATURES
HISTORY
Abdominal Discomfort or Pain
IBS should not be diagnosed in the absence of abdominal discomfort or pain.3 Distinguishing discomfort from pain can be problematic for both the patient and physician, however, because of the strong influence of cultural issues. In the United States, what a physician might label as mild pain often is considered discomfort by the patient. The pain or discomfort in IBS typically is relieved by defecation, or its onset is associated with an increase or decrease in stool frequency, or looser or harder stools. The pain often is poorly localized, waxes and wanes, may be aggravated by eating, and can occur in any part of the abdomen, although it more typically is located in the lower abdomen; it may be referred to different areas in the abdomen or to the chest or back. Exacerbation of pain by life events or difficult life situations is common. Abdominal discomfort or pain that is continuous or unrelated to defecation or induced by menstruation, urination, or physical activity is unlikely to be caused by IBS.
Constipation and Diarrhea
Patients with IBS experience constipation, diarrhea, or a mixture of these symptoms.3 Symptom predominance has led some authors to attempt to classify IBS patients by their predominant symptom: constipation (IBS-C), diarrhea (IBS-D), and mixed (IBS-M), although these symptoms often are variable and intermittent and patients can change their bowel habits from one pattern to another (see later). An irregular stool consistency (abnormal stool form) is characteristic. The terms “constipation” and “diarrhea” can reflect a wide variety of different symptom experiences to different patients, and so whenever a patient uses these terms, an exploration of their meaning is required.18 Any combination of infrequent defecation, passage of hard stools, excessive straining, feelings of incomplete rectal evacuation, or rectal discomfort may be referred to as constipation, whereas increased stool frequency, urgency, or the passage of liquid or watery stools, or even more frequent small hard stools, may be referred to as diarrhea by the patient. Stool form can be measured objectively and graded by patient or physician; the Bristol stool form scale (Fig. 118-1) now is routinely used in clinical trials, and changes in stool form (at the extreme ends of the scale) roughly correlate with colonic transit time.19,20
Bloating and Visible Distention
A feeling of bloating is almost universal in patients with IBS, and its site can be difficult for the patient to localize. Visible abdominal distention is characteristic but less common21; it can be objectively measured and usually is not imagined.22 Gas can mean excess bloating, belching, flatus, or even reflux symptoms to the patient, and so it is important to ask patients to explain the meaning of the terms they are using to describe their symptoms.
Noncolonic Symptoms
Other clinical features can help support the diagnosis of IBS but in themselves are not diagnostic. Nausea is common, and at least one third of patients with IBS have epigastric discomfort or pain (dyspepsia).1,8,23 Gastroesophageal reflux disease (GERD) occurs more commonly in IBS than would be expected by chance, affecting up to one in three persons with IBS.24 Extraintestinal symptoms including headache (and migraine), backache, impaired sleep, chronic fatigue, increased urinary frequency or urgency, pelvic pain, and dyspareunia are more common in patients with IBS but have no accepted diagnostic value.1,25 Musculoskeletal pain syndromes including fibromyalgia23 and temporomandibular joint disorder also are associated with IBS.2,23
Inflammatory Bowel Disease and Irritable Bowel Syndrome
Typical IBS symptoms are common in patients with documented inflammatory bowel disease (IBD) in remission; in one study, 33% with ulcerative colitis and 42% with Crohn’s disease fulfilled Rome II criteria for IBS.26 Clinically these conditions can be difficult to distinguish. IBS symptoms appear to be more prevalent before a diagnosis of IBD is made.27
Chronicity
For a confident diagnosis of IBS, symptoms should have been present for at least six months3; IBS may accompany other chronic disorders. For example, IBS is present in one third or more of patients with IBD in remission.26 A number of different conditions can cause transient bowel symptoms including pregnancy, dietary indiscretion, food poisoning, traveler’s diarrhea, bed rest, weight loss, and acute stress (nervous diarrhea); these must be distinguished from the chronic, recurrent symptoms of IBS.
PHYSICAL EXAMINATION
The physical examination in IBS usually is normal, although deep tenderness over the colon may be appreciated.10 Abdominal wall pain should be excluded clinically. Tensing the abdominal wall by flexing the chin on the chest or sitting up partially lessens tenderness that is caused by an intra-abdominal process. If tensing the abdominal wall muscles increases abdominal tenderness, a point of localized abdominal wall tenderness should be sought with a probing finger (Carnett’s test); identification of such a point might enable the tenderness to be treated with an injection of lidocaine and triamcinolone.28,29 The painful rib syndrome (point tenderness on springing the rib cage) also may be confused with IBS pain.30 Ovarian cancer needs to be considered in any middle-aged or older woman presenting with new-onset IBS-like symptoms.31 A pelvic examination therefore may be relevant to determine if there is any irregular, fixed pelvic mass.
EPIDEMIOLOGY
IBS is a common disorder all over the world.1,17,32 Epidemiologic studies have defined the prevalence and identified potential risk factors for IBS.
PREVALENCE
Prevalence estimates for IBS have varied anywhere from 3% to 20% in the United States, with similar results reported elsewhere; however, prevalence estimates are influenced substantially by the definition applied. For example, in Olmsted County, Minnesota, the prevalence of IBS varied from 8% to 22% depending on the criteria used.33
Younger people have a higher prevalence of IBS in the community. Generally, it is believed that IBS is uncommon in the elderly, but population-based studies indicate that IBS increases with advancing age. Thus, for example, using three or more of the Manning criteria to define IBS, the prevalence of IBS in Olmsted County ranged from 8% in those 65 to 74 years of age to more than 12% in those older than 85 years.34 Obviously, organic disease is more prevalent in elderly persons and could account for some of the reported IBS-like symptoms, but it seems likely that IBS in the elderly is often underdiagnosed or misdiagnosed, for example, as diverticular disease.35
GENDER AND RACE
Gender-specific prevalence rates for IBS are approximately two female to one male in most studies, and all population-based studies have reported a female predominance.17,36 Healthy women have greater rectal sensitivity, slower colonic transit, and smaller stool outputs than do men, which might explain why certain symptoms, such as straining and passage of hard stools, seem to be more common in women.37,38 In clinical practice in the United States, women outnumber men, which partly is explained by increased health care–seeking behavior among women; this appears to be culturally derived, because data from India indicate more men than women present for care of IBS in that part of the world.39
The prevalence of IBS generally is similar in whites and blacks, although some data have suggested it may be lower in Hispanics than in non-Hispanic whites in the United States.40,41 IBS is common in China, Japan, South America, and the Indian subcontinent. Indeed, IBS is common and its prevalence comparable in all countries where it has been studied.39,42–45
SUBGROUPS
Subdividing IBS based on the predominant symptom pattern is attempted commonly, but few data are available on IBS prevalence by symptom subgroup. Moreover, it is unclear if those with one predominant symptom—diarrhea or constipation—if followed long enough, eventually develop the other, namely, constipation in patients with IBS-D or diarrhea in patients with IBS-C; some data from primary care support this contention.46 The Rome III definition uses stool form to subclassify IBS, but definitions of IBS subgroups remain arbitrary, and different definitions have been used in different studies (see Fig. 118-1).3 Nonetheless, in a study from Olmsted County, Minnesota, 5.5% of the population had IBS-D and 5% IBS-C; both diarrhea and constipation-type symptoms occurred in 5% (IBS-M), and 4% did not meet strict criteria for either constipation or diarrhea.47 Another population-based study found higher rates of constipation in community subjects with IBS.48 In a study of 317 patients recruited for a clinical trial, at baseline 36% had IBS-D, 34% had IBS-C, and 31% had IBS-M49; more than 75% switched type over a year of follow-up, usually to and from the IBS-M pattern, but less than a third changed from IBS-D to IBS-C or vice versa.49
INCIDENCE AND DISAPPEARANCE OF SYMPTOMS
The onset rate of IBS was 67 per 1000 person-years by applying the Manning criteria to a cohort in Olmsted County that was surveyed at a 12- to 20-month interval.50 This study did not exclude people with a past history of IBS, however, and hence this is not the true incidence.50 Another study reported that the incidence of a clinical diagnosis of IBS in Olmsted County was 0.2% per year; this figure reflects the lower end of the incidence rate, because people with IBS symptoms who did not seek consultation could not be included in this calculation.51 Over a 10-year follow-up, 15% of community subjects free of baseline IBS symptoms developed the syndrome.32
In a follow-up study in Olmsted County, 38% of subjects meeting the definition of IBS at entry did not meet these criteria 12 to 20 months later50; they lost their symptoms. The actual prevalence of IBS did not change from year to year, however, because the disappearance of symptoms in some patients with IBS was balanced by others who developed IBS. Among those losing IBS in the community, there is a subset in whom symptoms evolve to reflect another functional gastrointestinal disorder52,53; hence, IBS usually is a chronic disorder, although symptoms often are variable.
RISK FACTORS
The best-accepted risk factor for IBS is bacterial gastroenteritis.54–57 The risk of postinfection IBS has been reported to be increased with depression,58 adverse life events and hypochondriasis,59 female gender, younger age, and prolonged duration of diarrhea following the initial attack.60 Bacterial virulence factors also may be important,61 but IBS can follow nonbacterial enteritis, including Norovirus gastroenteritis, or infection with trichinella.62
Other risk factors for IBS include an affluent childhood environment,63 estrogen use, postmenopausal estrogen use,64 recent antibiotic use,65 food intolerance,66,67 extraintestinal somatic symptoms,67 and poor quality of life.32 IBS runs in families,68 and low birth weight is also a risk factor for IBS, even after controlling for genetic influences.69 In contrast, oral glucocorticoid users may be at a lower risk for IBS.70 IBS is associated with an approximately three-fold increased risk of ischemic colitis71; however, a cause-and-effect relationship has not been established and the absolute risk remains very small (43 per 100,000 person-years).
HEALTH CARE SEEKING
Understanding why a patient is presenting for care is important in terms of planning appropriate management strategies. Burden of illness studies estimate that there are 3.6 million physician visits for IBS in the United States annually.72 The rate of health care seeking for IBS may, in part, be affected by health care access; consulting rates in the United States have varied between 25% and 46%, but up to 40% of patients do not have easy access to health care in this country.73 In Australia, where health care access essentially is universal, consulting rates have been 73%.73
Drivers of health care seeking remain poorly documented.73 The severity and chronicity of symptoms, in particular abdominal pain, partly promote health care seeking.74,75 IBS patients are more concerned about their health and more fearful of illness, suggesting that anxiety about their illness may be another factor.73 Children of a parent with IBS may see physicians more often than those who do not have a parent with IBS.76 As adults, they also are more likely to report poorer health in childhood as well as having received greater parental attention and gifts or rewards for being ill, suggesting there may have been early childhood programming of abnormal illness behavior.77 Those who seek medical attention tend to be more disturbed psychologically than those who do not seek such consultation,78–81 and those who consult for IBS also are more likely to consult about relatively minor complaints as well as other nongastrointestinal somatic symptoms.73 There remain other unknown factors that must be important, however, because these psychological factors still seem to poorly explain observed health care–seeking rates.73
EXCESS ABDOMINAL SURGERY
There is evidence that patients with IBS are at risk for undergoing excess surgery.82–84 In a large health maintenance organization study, patients with IBS, compared with controls, reported having had more cholecystectomies (12% vs. 4%), appendectomies (21% vs. 12%), and hysterectomies (33% vs. 17%); IBS was associated independently with these operations.82 A full explanation for these findings is uncertain, but presumably, some of this excess surgery reflects misdiagnosis and inappropriate intervention.85 It also is possible that IBS could predispose to an excess of certain diseases that lead to surgery. For example, constipation is associated with an increased risk of gallstones,86 but whether this association applies to IBS-C is uncertain. Alternatively, a history of a biliary event—identification of gallstones or a cholecystectomy—is associated with an increased risk of new-onset IBS.87 Some surgeons continue to believe that patients with IBS-type symptoms respond favorably to intra-abdominal surgery, although the evidence is anecdotal and probably reflects a placebo response.85
IMPACT ON QUALITY OF LIFE AND COSTS
A systematic review concluded that there was good evidence for a decrease in health-related quality of life in patients with moderate to severe IBS88 and that the quality of life in IBS is impaired to a degree comparable with other chronic disorders such as depression or GERD. Rather than IBS causing impaired quality of life, an alternative explanation for this association is that poor quality of life predisposes to a higher risk of IBS, and some evidence supports this contention.32 Regardless, the presence of impaired quality of life in IBS indicates that IBS deserves serious attention and therapeutic intervention.88
IBS is associated with substantial costs because of days lost from work, excess physician visits, diagnostic testing, and use of medications.89–91 Patients with IBS miss three times as many days from work as do those without bowel symptoms.92 IBS is the sixth leading physician diagnosis in outpatients in the United States, and this is likely an underestimate.93 A comprehensive burden-of-illness study in the United States estimated that IBS cost $1.6 billion in direct costs and a staggering $19.2 billion in indirect costs.72 Moreover, patients with IBS consume over 50% more health care resources than do matched controls without IBS.89
PATHOPHYSIOLOGY
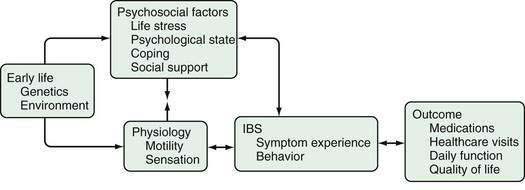
A number of different mechanisms have been implicated in the pathogenesis of IBS, including abnormal motility, visceral hypersensitivity, low-grade inflammation, and stress.1,8,94 Genetic factors could modulate the processing of gastrointestinal signals centrally and the inflammatory and immune responses locally, possibly predisposing to IBS. It seems reasonable to postulate that for IBS to manifest, several abnormalities—multiple hits—might need to occur. Some authors, therefore, conceptualize IBS as “a discrete collection of organic bowel diseases,”8 whereas other experts are concerned about “organification” of IBS because it might reduce the emphasis on the biopsychosocial model1,95 and imply that biological factors are sufficient to cause the disease. It seems likely that in IBS, an understanding of the individual, including his or her psychosocial nature and response to environmental factors, influences the expression of any biological determinants (Fig. 118-2). Regardless, further major therapeutic advances in the field seem unlikely to occur until the specific biological basis for symptoms is better identified.
ALTERED COLONIC AND SMALL BOWEL MOTILITY
In IBS, diarrhea can occur from multiple colonic mechanisms including increased high-amplitude propagated contractions (HAPCs), an enhanced gastrocolic response (prolonged rectosigmoid motor activity in response to a meal), or rectal hypersensitivity.96–98 Constipation may be secondary to increased segmental (nonpropulsive) contractions, decreased HAPCs, or reduced rectal sensation.99–101 Colonic and small bowel transit has been documented to be delayed in IBS-C and accelerated in IBS-D.1,101,102
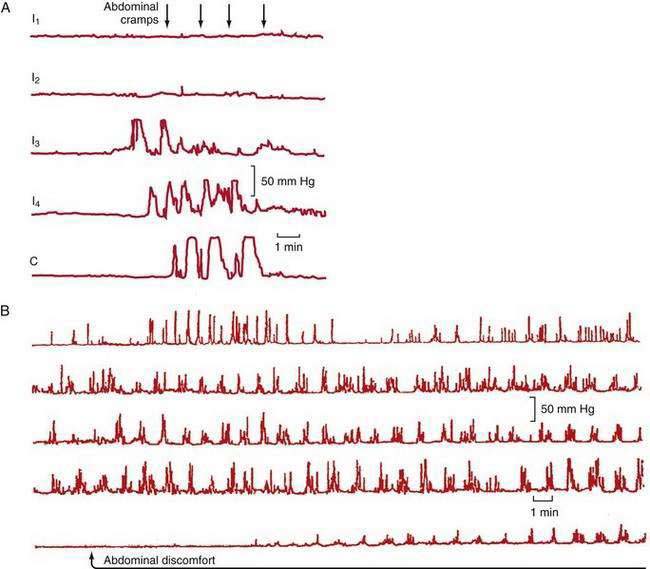
Abdominal pain in IBS also may be associated with HAPCs.103 A greater increase in phasic contractions in the terminal ileum and colon has been observed following distention, fatty meals, and cholecystokinin in patients with IBS (Fig. 118-3A).104 Discrete clusters of jejunal contractions also have been noted with increased frequency and duration in IBS (Fig. 118-3B), and they have been associated with pain in limited numbers of patients with IBS.104
Colonic motility in IBS can be increased by stress, anger, or instillation of deoxycholic acid, but this increase, although greater than in controls, is not specific for IBS1; a greater increase in colonic phasic contractions has been observed after administration of corticotropin-releasing hormone (CRH),105 and this increase is reduced by a CRH antagonist.106 Patients with IBS also have greater small bowel motor stimulation than do controls after cholecystokinin infusion, a fatty meal, or ileal distention.107 Autonomic dysfunction also has been reported in IBS patients with sympathetic adrenergic dysfunction associated with diarrhea and vagal dysfunction with constipation.108
VISCERAL HYPERSENSITIVITY
In the 1970s, balloon distention in the rectum was shown to induce pain at lower volumes in patients with IBS109; this has been confirmed in multiple studies using the barostat balloon that controls for changes in compliance, leading to the suggestion that colonic hypersensitivity is a useful biological marker of IBS.110,111 Visceral hypersensitivity might explain the fact that IBS patients seem more likely than controls to be aware of the presence of gas or intestinal contractions after meals or stress. Visceral hypersensitivity probably is confined to the intestine because somatic pain thresholds are normal, although not all studies agree on this.112–114
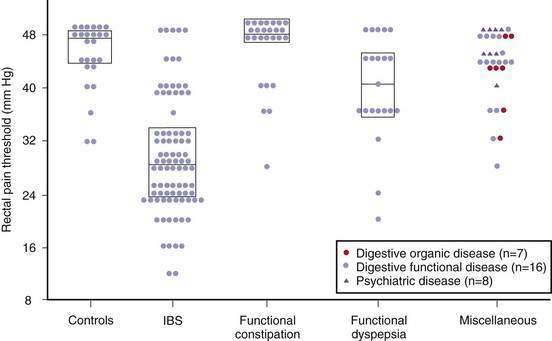
Visceral hypersensitivity is not a universal finding in patients with IBS and affects about 60% of patients (Fig. 118-4).115 In contrast to control subjects, patients with IBS and normal baseline visceral hypersensitivity might have rectal hypersensitivity induced by repeated distention of the sigmoid colon.116 This suggests that in IBS there is abnormal sensitization within the dorsal horn of the spinal cord or higher up in the central nervous system.
Putative neurotransmitters that are of relevance to visceral hypersensitivity include serotonin, neurokinins, and calcitonin gene-related peptide.117 The capsaicin (red pepper) receptor on nerve fibers, also called transient receptor potential vanilloid-1 (TRPV1), appears to be increased in the rectosigmoid colon in IBS and might mediate visceral pain.118 The N-methyl-d-aspartate (NMDA) receptor also may be important, because it modulates central (spinal cord) neuronal excitability.119 Visceral sensitivity, at least in the esophagus, can be reduced by an NMDA receptor antagonist.119
Serine proteases are thought to act as signaling molecules via the activation of proteinase-activated receptors (PARs). Extracts derived from colonic mucosal biopsies of patients with IBS (but not controls) have been observed to sensitize murine nerves in culture; this was blocked by a serine protease inhibitor.120 A significant increase in serine proteases has been observed in the stools of patients with IBS-D.121 Serine proteases could potentially damage tight junctions and increase intestinal permeability via PAR activation.122 The origin of stool serine proteases is uncertain, but they might derive from mast cells or the fecal microbiota.122
It is possible that inflammation is responsible for the sensitization in a subset of patients with IBS, as discussed later; however, some of this decreased threshold to balloon distention may be attributed to hypervigilance or excessive attention to, or fear of, a painful stimulus.123
ABNORMAL GAS PROPULSION AND EXPULSION
Ambulatory monitoring of abdominal girth has revealed that the abdomen normally swells during the day, peaking in the late evening, but decreasing on lying down; this phenomenon often is exaggerated in IBS.124 Retention of gas following gas infusion into the small intestine is greater in patients with IBS than it is in healthy controls.125 Furthermore, in those with IBS, intestinal gas infusion induces more discomfort than it does in controls when subjects are asked to voluntarily suppress passing the gas.125 During gas infusion, IBS patients, in contrast to healthy controls, involuntarily suppress their abdominal wall muscle contraction, which might explain their tendency to become distended; this could reflect an abnormal intestinal-somatic reflex response.126
Small intestinal bacterial overgrowth (SIBO) has been speculated to contribute to bloating in IBS, but this is not established with certainty.127,128 Although modest increases of bacteria have been documented in the proximal small intestine in IBS, SIBO rates do not differ between patients with IBS and controls.129 Similarly, lactulose breath testing, a surrogate marker of SIBO, often is abnormal in IBS using published criteria, but careful studies in controls suggest the rates of abnormality do not differ.130 Methane gas production by methagenic fecal flora, which occurs in the minority of the population, is now well established to be associated with constipation,128,131 possibly via slowing of intestinal transit in predisposed persons.132
Intravenous neostigmine has been demonstrated to clear retained intestinal gas and to reduce abdominal symptoms in patients with IBS and functional bloating.133 Physical activity might also enhance gas transit,134 and thus is to be encouraged.
LOCAL INFLAMMATION
The normal intestine is chronically in a state of inflammation, which occurs because of the balance between commensal enteric organisms and the host immune system. Inflammatory cells, including mast cells,135–138 and activated T lymphocytes139,140 are increased above normal in the mucosa in a subset of patients with IBS, suggesting that a low-grade inflammatory bowel disease may be present. Furthermore, lymphocytic infiltration of the myenteric plexus associated with neuron degeneration has been observed in severe IBS,141 as have increased mast cells in the muscularis externa.142 The cause of these abnormalities is unknown, but infections, abnormal bacterial flora, bile, or food antigens all could be contributors.
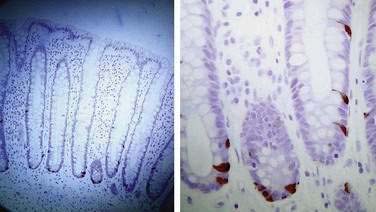
From 7% to 30% of patients who have recovered from a proved episode of bacterial enteritis develop IBS.54–56,58,60,61 One study, however, has suggested that those with pre-existing IBS who develop gastroenteritis may be more likely to seek medical consultation, thereby inflating the apparent risk estimates of this group.143 If the illness lasts more than three weeks or there are organisms involved that are toxigenic, then the risk of postinfection IBS is increased.61 Furthermore, those with psychological distress might have a further increased risk of postinfection IBS.55,58,59 In those who develop postinfection IBS, there are increases in CD3, CD4, and CD8 T lymphocytes, macrophages, and enteroendocrine (enterochromaffin) cells (Fig. 118-5).58,139 Increased small intestinal permeability as demonstrated by the lactulose-mannitol test also has been reported to occur in postinfection IBS61,139; however, this test is confounded by intestinal transit and bacterial overgrowth, and whether abnormal intestinal permeability occurs remains speculative.
Mast cells may play a central role in IBS. Activated mast cells release tryptase and histamine and have been observed to lie in close proximity to colonic nerves in patients with IBS; this finding has been correlated with abdominal pain.144 Supernatants prepared from colonic mucosal biopsies of patients with IBS have been shown to excite rat nociceptive visceral sensory nerves, suggesting that mast cell mediators, including tryptase, histamine, and prostaglandin E2, might represent another mechanism inducing visceral hypersensitivity in IBS.145
Colonic inflammation is associated with the production of a number of important mediators including 5-hydroxytryptamine (5-HT), prostaglandins, bradykinins, adenosine, and nerve growth factors.8 Abnormal release of 5-HT might have a central role in the manifestations of IBS.146 More than 95% of 5-HT is located in the enteroendocrine cells of the intestine and 5-HT is released from these cells following stroking or increased pressure, such as after a meal; 5-HT then acts on primary intrinsic afferent neurons to initiate the peristaltic reflex by activation of ascending excitation and descending inhibition.8,147 5-HT is taken up again by a specific serotonin transporter (SERT) expressed in the enterocytes. There is some evidence that an exaggerated release of 5-HT in IBS can occur after a meal.148
It has been observed in rectal biopsy specimens that 5-HT molecular signaling may be abnormal in IBS. In one study, 5-HT reuptake was reduced in IBS and also in ulcerative colitis compared with controls, although 5-HT release was unaffected and the numbers of enteroendocrine cells were unchanged.149 The findings were similar in IBS patients with constipation or diarrhea, leading to the hypothesis that in IBS there is increased availability of mucosal 5-HT that can induce diarrhea, but if there is desensitization of 5-HT receptors, this leads to constipation or an alternating bowel pattern149; confirmatory data currently are not available.150
ROLE OF FOOD
Many patients with IBS attribute their symptoms to certain foods, with wheat, dairy products, citrus fruits, potatoes, onions, and chocolate most commonly implicated.151,152 In an uncontrolled trial, one half of the patients with IBS reported improvement with elimination diets.66
Wheat Intolerance or Allergy
Substantial amounts of wheat are eaten in Western countries, 10% to 15% of which is not digested by human enzymes.8 Furthermore, subtle forms of gluten intolerance may be present in some people with IBS who do not have any overt evidence of celiac disease (see Chapter 104).153 A six-month gluten-free diet improved symptoms in 70% of patients with IBS-D who were HLA DQ2 positive,154 compared with 20% who were negative. In the absence of a definitive diagnosis of celiac disease, however, a gluten-free diet remains of unproven benefit.8
Sugar Malabsorption
Symptoms of IBS can be confused with those of lactose intolerance.155 Lactose intolerance occurs with varying prevalence depending on one’s ethnic group and is seen in 10% of populations of northern European descent, 40% to 60% of those of Asian descent, 90% of Chinese, and 60% to 80% of Africans (see Chapter 101). In acquired hypolactasia, there is some residual ability to digest small amounts of lactose and because most people do not ingest more than 12.5 g of lactose a day, they do not suffer from this ingestion.156 Unless a lactose-intolerant patient regularly ingests substantial amounts of lactose, lactose intolerance cannot be the explanation for the symptoms.157
Fructose and sorbitol malabsorption might contribute to IBS symptoms in some patients; however, fructose and sorbitol malabsorption, with a prevalence of 30% in those with IBS, may be no more common in IBS than in the background population.158–162 In a double-blind rechallenge trial, 25 patients who had responded to fructose withdrawal were challenged with fructose or fructans; nearly 80% developed symptoms compared with less than 15% who were given glucose.161
ABNORMAL COLONIC FLORA AND SMALL INTESTINAL BACTERIAL OVERGROWTH
It has been suggested that the colonic flora could be abnormal in a subset of patients with IBS, resulting in increased colonic fermentation, production of excess gas, and development of symptoms.163 This has led to an interest in pre- and probiotic therapy for IBS.
Others have reported that there is a high prevalence of SIBO in IBS, based on hydrogen breath testing and the clinical response to nonabsorbable antibiotics.164,165 Abnormal hydrogen breath test results, however, can occur because of transit abnormalities, and any association with IBS remains to be established.130,166,167
CENTRAL DYSREGULATION
Visceral afferent signals from the intestine reach the brainstem and thalamus and are consciously perceived only occasionally, although there may be some subliminal registration of low-intensity signals.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree